Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 3 by Beatrix Zheng.
This is an overview of polyhydroxyalkanoate (PHA)–vegetal fiber composites, the effects of the fiber type, and the production method's impact on the mechanical, thermal, barrier properties, and biodegradability, all relevant for biopackaging. To acknowledge the behaviors and trends of the biomaterials reinforcement field, the researchers searched for granted patents focusing on bio-packaging applications and gained insight into current industry developments and contributions.
- polyhydroxyalkanoates
- fibers
- mechanical properties
- biodegradability
- packaging
- patents
1. Polyhydroxyalkanoate and Fiber Composites
Natural fibers are mainly composed of cellulose, hemicellulose, and lignin, which have different physical and chemical properties [1][2]. Different natural fibers have been used to reinforce polyhydroxyalkanoates (PHAs), but their inclusion also presents some issues. For example, cellulose provides the strength of the fiber but has poor compatibility with hydrophobic polymers such as PHA [3][4]. Hemicellulose is amorphous and hydrophilic due to hydroxyl and acetyl groups; therefore, its mechanical properties are poor, and it retains moisture [5][6]. The lining is aromatic and amorphous but less hydrophilic than other components [4][5]. These characteristics cause low interfacial adhesion between fiber and matrix and the generation of polar groups, which generate poor dispersion in the matrix [7][8]. Thus, different pretreatments have been used to reduce the polarity and water absorption of fibers to improve the affinity between the fillers and the matrix and enhance the efficient stress transfer from matrix to fibers.
With pretreatments of chopping or grinding, fibers are cut by mechanical methods and sieving, or micronized, to obtain smaller particle sizes to improve fiber–matrix adhesion and promote crystallization [9]. Grinding methods include cutting milling, impact milling, or ball milling [10]. Enzymatic pretreatment immerses the fibers in a pectinase, laccase, or cellulase solution to modify the fillers’ surfaces and remove impurities [11]. In grafting, powder cellulose fibers undergo an esterification process and are subsequently dried. These fibers are further treated to increase their hydrophobicity [12]. In mechanical–hydrothermal pretreatment, fibers are immersed in warm water for surface modification and bacterial degradation [13][14]. Mechanical–chemical pretreatment consists of grinding the vegetal fillers followed by an alkali or solvent treatment to remove impurities and to improve the fiber–matrix adhesion [15]. Lastly, an argon plasma jet induces new functional groups on the cellulose surface in the plasma pretreatment, allowing the fibers to ultrasonicate and lyophilize [16]. Additional reports on pretreatment of the fiber with waxes suggest performance improvements without hindering the biodegradability of the composite. For instance, in a blend of PHBV, wheat, ATBC, and calcium carbonate, the pretreatment of the fiber with bio-based waxes improves the mechanical performance of the blend in terms of impact resistance, and in a composite of PHBV–potato–ATBC–calcium carbonate, the wax pretreatment of the fiber enhances the fiber–matrix adhesion and the mechanical properties of the composite [17][18].
2. Mechanical Properties of PHA–Vegetal Fiber Composites
In general, the composite polymers use less than 30 wt% of reinforcements, probably due to the difficulty in achieving a homogeneous dispersion of the vegetable fibers, and melt flow index also decreases, which hinders composites’ processability. Young’s modulus, tensile strength, and elongation at break are the most commonly reported mechanical properties, typically measured using the ASTM D638 Standard Test Method for Tensile Properties of Plastics. In addition, ASTM D790 for Flexural Test of Plastics, ASTM D256 2018 Standard Test Methods for Determining the Impact Resistance of Plastic Izod Pendulum, and ISO 527 Determination of Tensile Properties in Plastic Films are used. After analyzing the literature and organizing the information according to the matrix materials and fibers used as reinforcement in composites, the reseachers plotted the reported data, regardless of the preparation method, to acknowledge behaviors and trends of the biomaterial reinforcement using Tableau (Salesforce company) for visual analysis (Figure 1, Figure 2 and Figure 3).
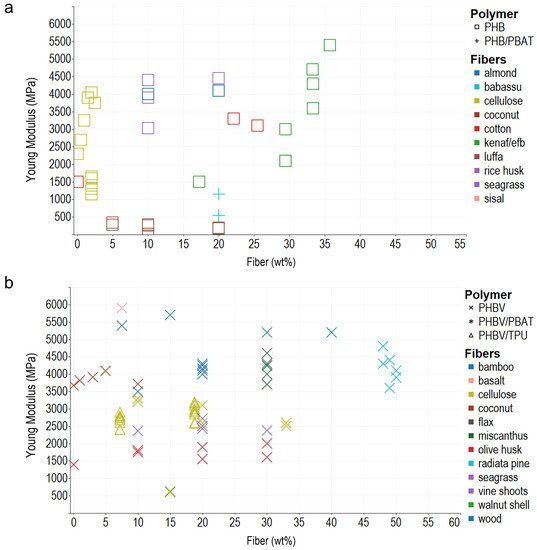
Figure 1. Young modulus of different PHA–fiber composites. (a) PHB and PHB/PBAT blends. (b) PHBV and its blends with PBAT and TPU. The colors refer to the fiber type (fillers), and the shapes refer to polymer types (matrix).
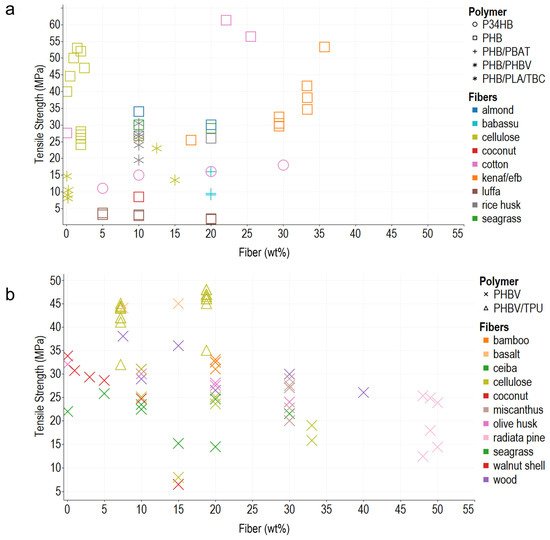
Figure 2. Tensile strength. (a) PHB and PHB/PBAT blends. (b) PHBV and its blends with PBAT and TPU. The colors refer to the fiber type (fillers), and the shapes refer to polymer types (matrix).
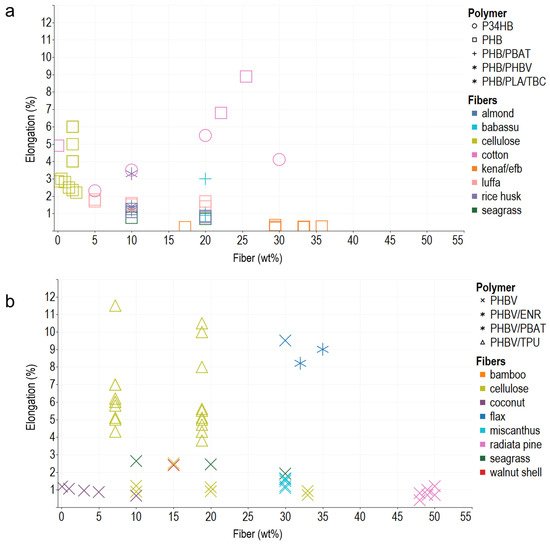
Figure 3. Elongation at break of PHA–fiber composites. (a) PHB and its blends with PBAT, PHBV, PLA, and TBC. (b) PHBV and its blends with ENR, PBAT, and TPU. The colors refer to the fiber type (fillers), and the shapes refer to polymer types (matrix).
3. Thermal Properties of PHA–Fiber Composites
Two main techniques determine transition temperatures to characterize polymers. In differential scanning calorimetry (DSC), the specimens are subjected to two heating cycles. The first heating process has the objective to erase the thermal history of the polymer matrix and remove moisture because water acts as a plasticizer and modifies the properties of the polymers, and the second cycle identifies melting and crystallinity temperature, and, in some cases, the generation of crystals of different sizes [19][20]. Thermogravimetric analysis (TGA) measures the mass variation when the temperature changes [21][22].
Figure 4 shows the relationship between fiber percentage and melting and degradation temperatures. Fiber addition does not significantly modify the material’s melting temperature, as seen in the compounds PHB–pissaba, PHB–rice husk, and PHBV–cellulose. This behavior is desirable since inexpensive vegetable fillers can be used in composites to reduce the cost without significantly affecting processability. Thermal stability is critical for packaging applications because some containers are exposed to high or low temperatures during shipping and storage. A biocomposite must endure heating or cooling processes [23][24][25].
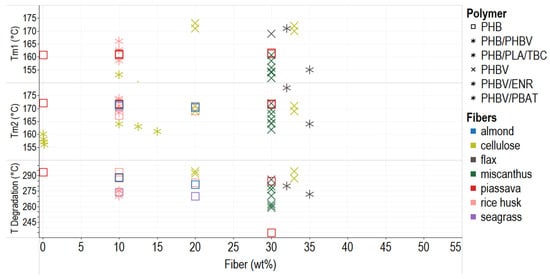
Figure 4. Thermal behavior of PHA–fiber composites. The colors refer to the fiber type (fillers), and the shapes refer to polymer types (matrix). Tm = temperature of melting.
The TGA shows that the degradation of composites PHA-vegetal fibers occurs in two main steps: first, the initial fiber and PHA degradation by hydrolysis, and second, the lining and residue degradation at 350 °C or more [26][27]. The addition of fibers also implies the addition of impurities, and the initial temperature of degradation (Tdeg) decreases; also, some interaction between fibers and PHBV matrix results in lower Tdeg of composites [28]. Fiber addition also causes differential melting temperatures in the first heating (Tm1) and the second cycle (Tm2) due to perfect crystal formation because more giant and more ordered crystals need more energy to melt again [9][29]. This behavior has been reported for PHB–flax, PHBV/ENR–flax, or PHBV–miscanthus composites (Figure 4). The degradation temperature of the composites decreases when using more significant amounts of the filler and additional treatments to reinforce, which implies less thermal stability.
Some treatments improve the thermal stability of composites due to the removal of pectin, cellulose, and other substances of the filler [30]; when fiber improves the interaction with the matrix, the thermal degradation is retarded [12]. Likewise, reactive agents impact the thermal behavior of composites. For example, DCP (>0.1 phr), the additive in a blend of PHBV–miscanthus (70–30 wt%). reduces the temperature of melting (Tm) of the blend by reducing crystallinity [31]. In some cases, additives mask the nucleating effect of the vegetable fiber in the polymeric matrix. In addition, plasticizers, typically used for internal lubrication, increase mobility and decrease the temperature of glass transition (Tg) [32]. The loss of the plasticizer usually appears in the first part of a TGA curve. This behavior is typical in PHB composites with glycerol and triethyl citrate (TEC), among others [33].
4. Barrier Properties of PHA–Fiber Composites
The barrier properties are essential for packaging materials, especially in food and shelf applications [34][35]. If a composite absorbs water or oils or has a high gas and vapor permeability, it is unsuitable for preserving the organoleptic properties of the package content [36]. The addition of vegetal fibers to a polymer matrix increases the porosity and the number of polar groups that result in absorbing water. Moreover, poor interfacial adhesion creates zones that efficiently uptake water [37][38]. The moisture reduces the mechanical properties of the blend and increases biodegradation because the water migrates to the amorphous zones and leads to polymer chain scission. Pretreatment of the vegetable fibers would reduce moisture absorption. Pretreatments such as esterification, use of NaOH, or enzymatic reduction achieve better dispersion and adhesion of the filler into the matrix, reducing the hygroscopicity [30][39].
Noteworthily, the water vapor transmission (WVP) of PHA films is similar to polyethyleneterephtalate (PET) films [36], but it increases with the addition of vegetal fibers because of the crystallinity changes generated by the fillers [40]. The WVP of a composite, as a measure of water vapor uptake, depends on fiber amount, crystallinity decrement, and changes in the molecular weight of the matrix [12]. The type of fiber and its hygroscopicity also affects the water vapor permeability [41]. For example, adding low amounts of fiber (2%) in a PHB–cellulose composite improves the crystallinity, reducing the diffusion process.
5. Biodegradability
A biodegradable polymer undergoes biodegradation, a chemical process during which microorganisms that are available in the environment decompose materials into natural substances such as water, carbon dioxide, and methane [42]. As per the ASTM D6400 definition, compostable plastics must demonstrate proper disintegration during the composting, an adequate level of inherent biodegradation, and no adverse impacts to support plant growth [43]. Most materials are biodegradable, but their biodegradation process might take hundreds of years [44]. Therefore, one of the objectives of biodegradable plastic developers is to achieve this process within an appropriate life span according to the use of the material and its subsequent disposal.
There are different methods to measure a polymer’s biodegradability. The test selection depends on the organization (ASTM, EPA, ODEC, ISO), external conditions (aerobic, anaerobic, UV exposure), the environment (soil, marine water, compost), or their purpose (biodeterioration, assimilation, biofragmentation) [45][46]. Among the most common tests used to monitor biodegradation are weight loss, abiotic degradation, CO2 measurement, macromolecular weight loss, oxygen consumption rate, and anaerobic digestion (biogas production–weight loss) in compliance with ISO 15814:1999, ISO 17556 (2019), ASTM G160–12, and ASTM D6691 standards [47][48][49][50][51]. CO2 measurements with a respirometric test also help identify the material’s shelf life according to ASTM D5988-96. These standards require that the material biodegrades in a certain period and leaves no toxic residue in the soil. Exposure to the environment (i.e., temperature, moisture, microbial population, pH, oxygen content) affects the biodegradation of a polymer; thus, a material that degrades by microbial activity under industrial composting conditions may not degrade in other conditions [52].
Reinforcement with vegetal fibers is expected to improve the biodegradability of the already biodegradable PHAs. Instead, biodegradation depends on soil composition, fiber amount and pretreatment, material stiffness, and processing [37]. For instance, the PHBV–shoot vine composite biodegradation rate is 83%. The content of lignin and polyphenols makes biodegradation difficult, but pretreatment of the fibers raises the composite’s biodegradation to 97%. In a different example, PHB degradation ranged from 60 to 98%, depending on the method. PHB showed 64.3% degradation in 6 months using microbial fermentation in soil tests [53]. In a quasi-steady state, co-digestion of synthetic municipal primary sludge (SMWS) and PHB, after 45 days, exhibited approximately 80–98% conversion of PHB to biomethane [54]. In soil, P(3HB) specimens had been 60% degraded in 21 days, but they continued to degrade to 93% by day 35 [55]. For comparison, blending PHB with wood fiber yields conflicting results. The biodegradation of [P(3HB-co-3HHx)]/Kenaf during 48 days in mineral medium-soil reached 13%, whereas, in an aqueous-nutrient medium, it barely reached 2.4% [47]. Biodegradation of a composite of P(3HB-co-4HB)/wood under laboratory composting for 21 days could not be detected, but in an aqueous medium, it was 0.35% in two months. However, biodegradation was 35% per year in soil [48][50].
Additives also impact biodegradability. TPU (18–21 wt%) added to a PHBV–cellulose composite reduced the disintegration of the blend because the TPU covered the filler and interfered with the microorganism’s access. When using HMDI, the degradation rate increased because the plasticizer blocked the effect of the TPU on the fibers [41]. Likewise, bio-based plastics with additives tested in soil media for 660 days did not show significant biodegradation. Instead, the PHA film reported 70% mineralization, very similar to the cellulose control film measured under the same conditions [56]. In a PHBV–posidonia composite, using ATBC as a plasticizer increases the polymer chain mobility and accelerates the disintegration of the blend [57]. This research measured the specimen’s degradation in marine mesocosms and found degradation in warm seawater conditions. The specimens used in the test maintained their tensile properties by ten months, suggesting possible applications in marine ítems.
Disposal in landfills raises additional concerns for biodegradable plastics. A food packaging study observed that packaging made with biodegradable materials releases methane, more harmful than CO2 [58]. Thus, alternate bioremediation strategies are needed, such as using methanotrophic microorganisms to reduce methane emissions in landfills or using the methane for energy generation [59][60][61] to take full advantage of biopackaging waste biodegradation.
6. Theoretical Modeling to Evaluate Performance and Applications of Polymer–Vegetal Fiber Composites
Although blending with fibers improves the polymers’ mechanical properties, the extent of the fiber contribution is unknown. Theoretical modeling has helped infer the performance and modifications expected for these composites. PHA/hemp and PHA/jute (30 wt%) modeling showed damage on the matrix due to the different physical properties of the fillers. In this research, hemp was the best filler and achieved a better interface that supports higher mechanical loads [62]. A PHBV/oak wood flour composite was modeled using a modified Fickian diffusion law and the Halping-Tsai and Nicolas and Nicodemo model to predict composite properties, such as moisture absorption, stiffness, and strength. Despite the improved mechanical properties of the composite, the blend is susceptible to deterioration by the hygrothermal behavior of the fiber [63].
Further modeling and experimental testing are essential to make better predictions. A 3D model of PHBV–wheat straw designed to predict water vapor permeability using the finite element method (FEM) to the 3D structures permitted a better prediction of water vapor permeability dynamics of the composite [64]. Numerical homogenization and representative volume elements (RVEs) are used to model composites’ effective elastic, thermal, and thermoelastic properties. This methodology allows for the preservation of fiber–matrix interactions and the predicted effective properties of blends, which can be further validated with experimental data [65].
References
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.R.; Jones, C.L.; Huhnke, R.L. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour. Technol. 2012, 114, 663–669.
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63.
- Cazón, P.; Velázquez, G.; Vázquez, M. Regenerated cellulose films combined with glycerol and polyvinyl alcohol: Effect of moisture content on the physical properties. Food Hydrocoll. 2020, 103, 105657.
- Motaung, T.E.; Linganiso, L.Z. Critical Review on Agrowaste Cellulose Applications for Biopolymers; Springer India: New Delhi, India, 2018; Volume 22, ISBN 1258801892.
- Melo, J.D.D.; Carvalho, L.F.M.; Medeiros, A.M.; Souto, C.R.O.; Paskocimas, C.A. A biodegradable composite material based on polyhydroxybutyrate (PHB) and carnauba fibers. Compos. Part B Eng. 2012, 43, 2827–2835.
- Bousfield, G.; Morin, S.; Jacquet, N.; Richel, A. Extraction and refinement of agricultural plant fibers for composites manufacturing. Comptes Rendus Chim. 2018, 21, 897–906.
- Chilali, A.; Assarar, M.; Zouari, W.; Kebir, H.; Ayad, R. Mechanical characterization and damage events of flax fabric-reinforced biopolymer composites. Polym. Polym. Compos. 2020, 28, 631–644.
- Meng, D.C.; Shen, R.; Yao, H.; Chen, J.C.; Wu, Q.; Chen, G.Q. Engineering the diversity of polyesters. Curr. Opin. Biotechnol. 2014, 29, 24–33.
- Melendez Rodriguez, B.; Torres Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive melt mixing of poly(3-hydroxybutyrate)/rice husk flour composites with purified biosustainably produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) beatriz. Materials 2019, 12, 2152.
- Martino, L.; Berthet, M.A.; Angellier-Coussy, H.; Gontard, N. Understanding external plasticization of melt extruded PHBV-wheat straw fibers biodegradable composites for food packaging. J. Appl. Polym. Sci. 2015, 132, 1–11.
- Zhuo, G.; Zhang, X.; Jin, X.; Wang, M.; Yang, X.; Li, S. Effect of different enzymatic treatment on mechanical, water absorption and thermal properties of bamboo fibers reinforced poly(hydroxybutyrate-co-valerate) biocomposites. J. Polym. Environ. 2020, 28, 2377–2385.
- David, G.; Gontard, N.; Angellier-Coussy, H. Mitigating the impact of cellulose particles on the performance of biopolyester-based composites by gas-phase esterification. Polymers 2019, 11, 200.
- Santos, E.B.C.; Barros, J.J.P.; de Moura, D.A.; Moreno, C.G.; de Carvalho Fim, F.; da Silva, L.B. Da Rheological and thermal behavior of PHB/piassava fiber residue-based green composites modified with warm water. J. Mater. Res. Technol. 2019, 8, 531–540.
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538.
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49.
- Vizireanu, S.; Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Ionita, M.D.; Satulu, V.; Carpen, L.G.; Petrescu, S.; Birjega, R.; et al. Cellulose defibrillation and functionalization by plasma in liquid treatment. Sci. Rep. 2018, 8, 15473.
- Gigante, V.; Cinelli, P.; Righetti, M.C.; Sandroni, M.; Polacco, G.; Seggiani, M.; Lazzeri, A. On the use of biobased waxes to tune thermal and mechanical properties of polyhydroxyalkanoates-bran biocomposites. Polymers 2020, 12, 2615.
- Righetti, M.C.; Cinelli, P.; Mallegni, N.; Stäbler, A.; Lazzeri, A. Thermal and mechanical properties of biocomposites made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and potato pulp powder. Polymers 2019, 11, 308.
- Iggui, K.; Le Moigne, N.; Kaci, M.; Cambe, S.; Degorce-Dumas, J.R.; Bergeret, A. A biodegradation study of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/organoclay nanocomposites in various environmental conditions. Polym. Degrad. Stab. 2015, 119, 77–86.
- Tian, J.; Zhang, R.; Wu, Y.; Xue, P. Additive manufacturing of wood flour/polyhydroxyalkanoates (PHA) fully bio-based composites based on micro-screw extrusion system. Mater. Des. 2021, 199, 109418.
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Development and thermochemical characterization of an antioxidant material based on polyhydroxybutyrate electrospun microfibers. Int. J. Biol. Macromol. 2021, 183, 772–780.
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.G. Alternative optimization routes for improving the performance of poly(3-hydroxybutyrate) (PHB) based plastics. J. Clean. Prod. 2021, 318, 128555.
- Othman, S.H. Bio-nanocomposite materials for food packaging applications: Types of biopolymer and nano-sized filler. Agric. Agric. Sci. Proc. 2014, 2, 296–303.
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619.
- Olayil, R.; Arumuga Prabu, V.; DayaPrasad, S.; Naresh, K.; Rama Sreekanth, P.S. A review on the application of bio-nanocomposites for food packaging. Mater. Today Proc. 2021. in Press.
- Camargo, F.A.; Innocentini-Mei, L.H.; Lemes, A.P.; Moraes, S.G.; Durán, N. Processing and characterization of composites of poly(3-hydroxybutyrate-co- hydroxyvalerate) and lignin from sugar cane bagasse. J. Compos. Mater. 2012, 46, 417–425.
- Nair, S.S.; Kuo, P.Y.; Chen, H.; Yan, N. Investigating the effect of lignin on the mechanical, thermal, and barrier properties of cellulose nanofibril reinforced epoxy composite. Ind. Crops Prod. 2017, 100, 208–217.
- Vandi, L.J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Wood-PHA composites: Mapping opportunities. Polymers 2018, 10, 751.
- Kuciel, S.; Mazur, K.; Jakubowska, P. Novel biorenewable composites based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with natural fillers. J. Polym. Environ. 2019, 27, 803–815.
- Guo, Y.; Wang, L.; Chen, Y.; Luo, P.; Chen, T. Properties of luffa fiber reinforced PHBV biodegradable composites. Polymers 2019, 11, 1765.
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Reactive compatibilization and performance evaluation of miscanthus biofiber reinforced poly(hydroxybutyrate-co-hydroxyvalerate) biocomposites. J. Appl. Polym. Sci. 2017, 134, 1–10.
- El-Hadi, A.M. Increase the elongation at break of poly (lactic acid) composites for use in food packaging films. Sci. Rep. 2017, 7, 1–14.
- Hosokawa, M.N.; Darros, A.B.; Moris, V.A.D.S.; De Paiva, J.M.F. Polyhydroxybutyrate composites with random mats of sisal and coconut fibers. Mater. Res. 2017, 20, 279–290.
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the water resistance of nanocellulose-based films with polyhydroxyalkanoates processed by the electrospinning coating technique. Cellulose 2018, 25, 1291–1307.
- Seoane, I.T.; Luzi, F.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Enhancement of paperboard performance as packaging material by layering with plasticized polyhydroxybutyrate/nanocellulose coatings. J. Appl. Polym. Sci. 2018, 135, 46872.
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer structures based on annealed electrospun biopolymer coatings of interest in water and aroma barrier fiber-based food packaging applications. J. Appl. Polym. Sci. 2018, 135.
- David, G.; Michel, J.; Gastaldi, E.; Gontard, N.; Angellier-Coussy, H. How vine shoots as fillers impact the biodegradation of PHBV-based composites. Int. J. Mol. Sci. 2020, 21, 228.
- Wei, L.; Liang, S.; McDonald, A.G. Thermophysical properties and biodegradation behavior of green composites made from polyhydroxybutyrate and potato peel waste fermentation residue. Ind. Crops Prod. 2015, 69, 91–103.
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85.
- Tănase, E.E.; Popa, M.E.; Râpă, M.; Popa, O. PHB/Cellulose fibers based materials: Physical, mechanical and barrier properties. Agric. Agric. Sci. Proc. 2015, 6, 608–615.
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225.
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8.
- A European Strategy for Plastics in a Circular Economy. European Parliament. Available online: https://ec.europa.eu/transparency/documents-register/detail?ref=COM(2018)28&lang=en/ (accessed on 10 January 2022).
- Single-Use Plastics: New EU Rules to Reduce Marine Litter. Delegation of the European Union to Montenegro. Available online: https://eeas.europa.eu/delegations/montenegro_fa/45931/Single-use%20plastics:%20New%20EU%20rules%20to%20reduce%20marine%20litter/ (accessed on 10 January 2022).
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442.
- Eubeler, J.P.; Zok, S.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. TrAC Trends Anal. Chem. 2009, 28, 1057–1072.
- Joyyi, L.; Ahmad Thirmizir, M.Z.; Salim, M.S.; Han, L.; Murugan, P.; Kasuya, K.; Maurer, F.H.J.; Zainal Arifin, M.I.; Sudesh, K. Composite properties and biodegradation of biologically recovered P(3HB- co -3HHx) reinforced with short kenaf fibers. Polym. Degrad. Stab. 2017, 137, 100–108.
- Musioł, M.; Jurczyk, S.; Sobota, M.; Klim, M.; Sikorska, W.; Zięba, M.; Janeczek, H.; Rydz, J.; Kurcok, P.; Johnston, B.; et al. (Bio)Degradable Polymeric Materials for Sustainable Future—Part 3: Degradation Studies of the PHA/Wood Flour-Based Composites and Preliminary Tests of Antimicrobial Activity. Materials 2020, 13, 2200.
- Šerá, J.; Serbruyns, L.; De Wilde, B.; Koutný, M. Accelerated biodegradation testing of slowly degradable polyesters in soil. Polym. Degrad. Stab. 2020, 171, 109031.
- Chan, C.M.; Vandi, L.-J.; Pratt, S.; Halley, P.; Richardson, D.; Werker, A.; Laycock, B. Insights into the biodegradation of PHA / wood composites: Micro- and macroscopic changes. Sustain. Mater. Technol. 2019, 21, e00099.
- Iwańczuk, A.; Kozłowski, M.; Łukaszewicz, M.; Jabłoński, S. Anaerobic biodegradation of polymer composites filled with natural fibers. J. Polym. Environ. 2015, 23, 277–282.
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452.
- Jain, R.; Tiwari, A. Biosynthesis of planet friendly bioplastics using renewable carbon source. J. Environ. Heal. Sci. Eng. 2015, 13, 11.
- Benn, N.; Zitomer, D. Pretreatment and anaerobic co-digestion of selected PHB and PLA bioplastics. Front. Environ. Sci. 2018, 5, 93.
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial degradation of polyhydroxyalkanoates with different chemical compositions and their biodegradability. Microb. Ecol. 2017, 73, 353–367.
- Gómez, E.F.; Michel, F.C. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591.
- Seggiani, M.; Cinelli, P.; Balestri, E.; Mallegni, N.; Stefanelli, E.; Rossi, A.; Lardicci, C.; Lazzeri, A. Novel sustainable composites based on poly(hydroxybutyrate-co-hydroxyvalerate) and seagrass beach-CAST fibers: Performance and degradability in marine environments. Materials 2018, 11, 772.
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.A.; Laycock, B. Environmental impact of biodegradable food packaging when considering food waste. J. Clean. Prod. 2018, 180, 325–334.
- Chiemchaisri, W.; Chiemchaisri, C.; Boonchaiyuttasak, J. Utilization of stabilized wastes for reducing methane emission from municipal solid waste disposal. Bioresour. Technol. 2013, 141, 199–204.
- Behera, S.K.; Park, J.M.; Kim, K.H.; Park, H.-S. Methane production from food waste leachate in laboratory-scale simulated landfill. Waste Manag. 2010, 30, 1502–1508.
- Ghosh, P.; Shah, G.; Chandra, R.; Sahota, S.; Kumar, H.; Vijay, V.K.; Thakur, I.S. Assessment of methane emissions and energy recovery potential from the municipal solid waste landfills of Delhi, India. Bioresour. Technol. 2019, 272, 611–615.
- Kaouche, N.; Mebrek, M.; Mokaddem, A.; Doumi, B.; Belkheir, M.; Boutaous, A. Theoretical study of the effect of the plant and synthetic fibers on the fiber-matrix interface damage of biocomposite materials based on PHAs (polyhydroxyalkanoates) biodegradable matrix. Polym. Bull. 2021, 1–21.
- Srubar, W.V.; Billington, S.L. Nonlonear micromechanical modleing of hygrothermal effect on structural biobased composite materials. In Multiscale and Multiphysics Processes in Geomechanics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 189–192.
- Kabbej, M.; Guillard, V.; Angellier-Coussy, H.; Wolf, C.; Gontard, N.; Gaucel, S. 3D Modelling of mass transfer into bio-composite. Polymers 2021, 13, 2257.
- Deierling, P.E.; Zhupanska, O.I. Computational modeling of the effective properties of spatially graded composites. Int. J. Mech. Sci. 2018, 145, 145–157.
More
