Ecological stoichiometry considers how nutritional needs of organisms for basic body-building molecules shape their ecology. It asks how nutritional demands of organisms and supply of nutrients in their environments affects organisms growth, their interactions with the biotic and abiotic worlds and nutrient cycling in whole ecosystem.
The balance of energy and matter in ecological interactions
----------------------
Ecological stoichiometry considers how nutritional needs of organisms for basic body-building molecules shape their ecology. It asks how nutritional demands of organisms and supply of nutrients in their environments affects organisms growth, their interactions with the biotic and abiotic worlds and nutrient cycling in whole ecosystem.
-----------------------------
- ecological stoichiometry
- ecology
- ecosystem ecology
- nutrient cycling
- food web
- food chain
- nutritional ecology
1. Introduction
The quality of food resources is crucial for animal growth, development and population dynamics. Therefore,
The quality of food resources is crucial for animal growth, development and population dynamics. Therefore,
limitations are posed on organisms because of food scarcity in specific nutrients. These limitations may shape ecological interactions and affect cycling of nutrients in the ecosystem. To better understand nutrient cycling driven by nutritional constraints posed on organisms, their communities and populations the ecological stoichiometry framework was developed (Figure. 1). This research framework capitalizes on organisms to be composed of identical building blocks - atoms of chemical elements - even though they build remarkable diversity of organic molecules having diverse functions [1][2][3]
[1–3]
. During growth and development organisms assimilate all the building blocks needed to compose the adult body. The body is built of atoms in taxonomically specific proportion known as the organismal stoichiometry[2]
[2]
. The demand for resources gathered during juvenile growth is reflected in organismal stoichiometry of adult body and stoichiometric mismatch (Figure .2) may occur between the atomic composition of the body and food[1][4]
[1]
. Even toxic effects may in fact be caused by such stoichiometric mismatch rather than true toxic substances[5].
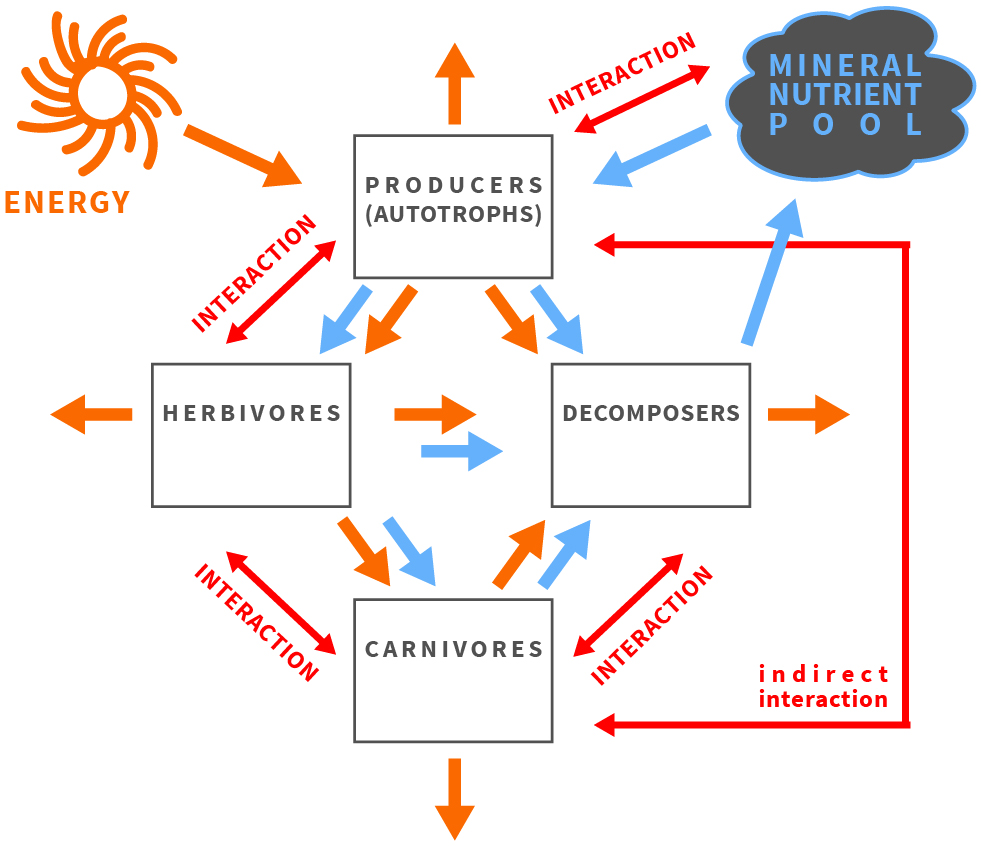
Figure 1. The balance of energy and matter affects and is affected by organisms and their interactions in an ecosystem.
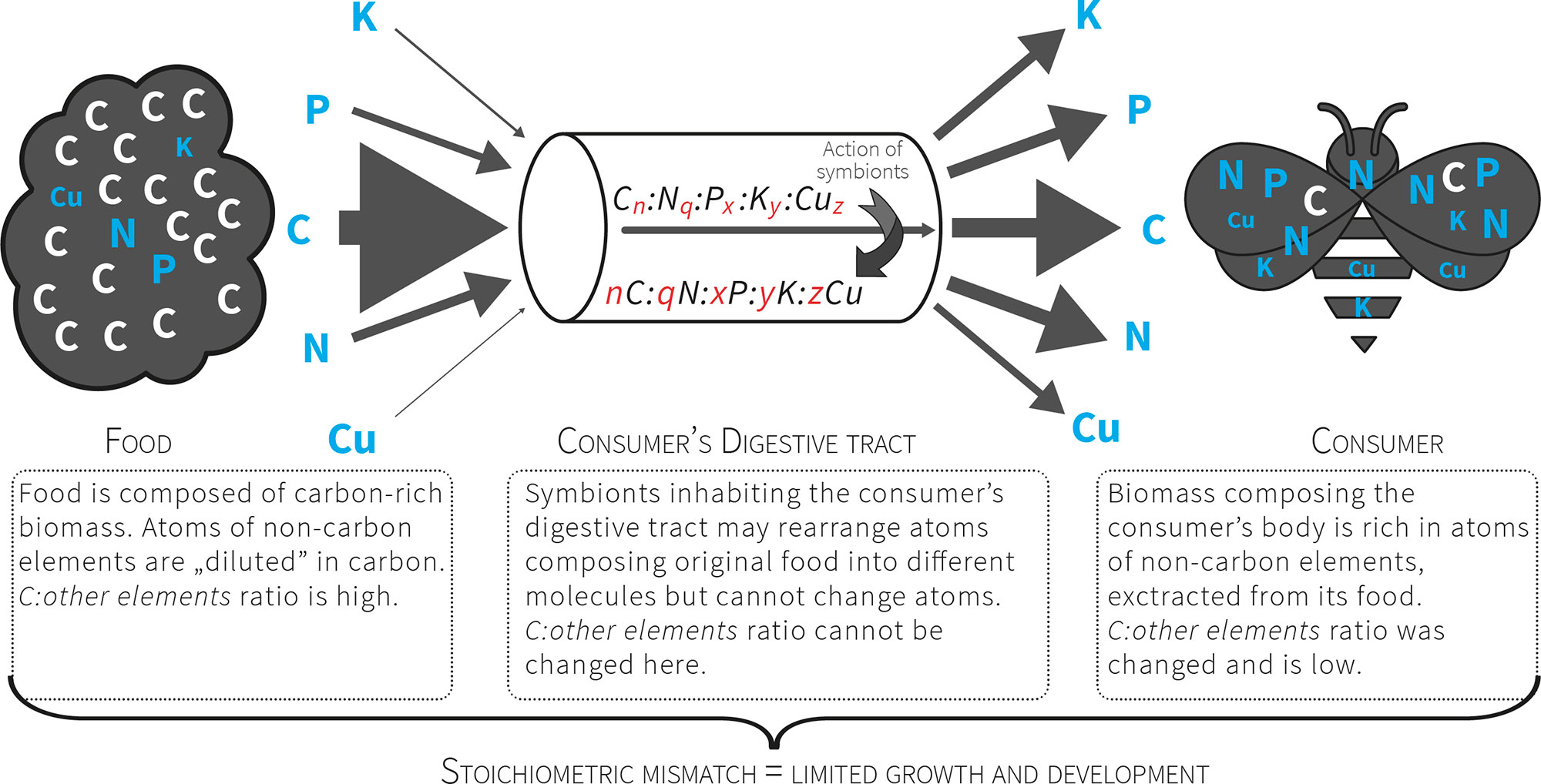
Figure 2 Consumers that feed on nutritionally poor food (e
[4]
.g. plant matter) consume a pre-packaged ratio of atoms. For herbivores the food contains more C relative to other atoms, so these organisms must manage a diet with excess C that presents a stoichiometric mismatch that limits their growth and development. The mismatch is further increased by unbalanced relation between non-C elements (exceptional scarcity of some of them).
2. History
The framework of ecological stoichiometry was envisioned in the works of Wiernadski[6][7] [5,6], Liebig, Playfair and Webster[8] [7], Lotka[9] [8], Tilman[10] [9] and Reiners[11] [10]; postulated explicitly as a fruitful approach by Sterner and Hessen[12] [11], Elser et al. [13][14][15][12–14]; and described in detail in the seminal book of Sterner and Elser[1] [1]. The synthesis written by Sterner and Hessen[12] [11], which focuses on nutrient limitations of aquatic herbivores, was recognized as a landmark paper that stimulated ecologists to broaden the understanding of producer-consumer interactions while considering ecological stoichiometry[16] [15]. The essential ideas and living-systems characteristics of ecological stoichiometry are as follows: (1) the law of conservation of mass and immutable atoms flowing through the food chain; (2) the capability of living organisms to transform organic substances; (3) stoichiometric stability (“homeostasis”), in which every species has a unique composition of chemical elements in the body tissues, with heterotrophs showing a lower level of variability than autotrophs; and (4) consumer-driven nutrient recycling (CNR), in which the flow of matter through the food chain is regulated by the elemental body composition of species, thereby composing particular links in the chain[1][4] ([1,16]).
3
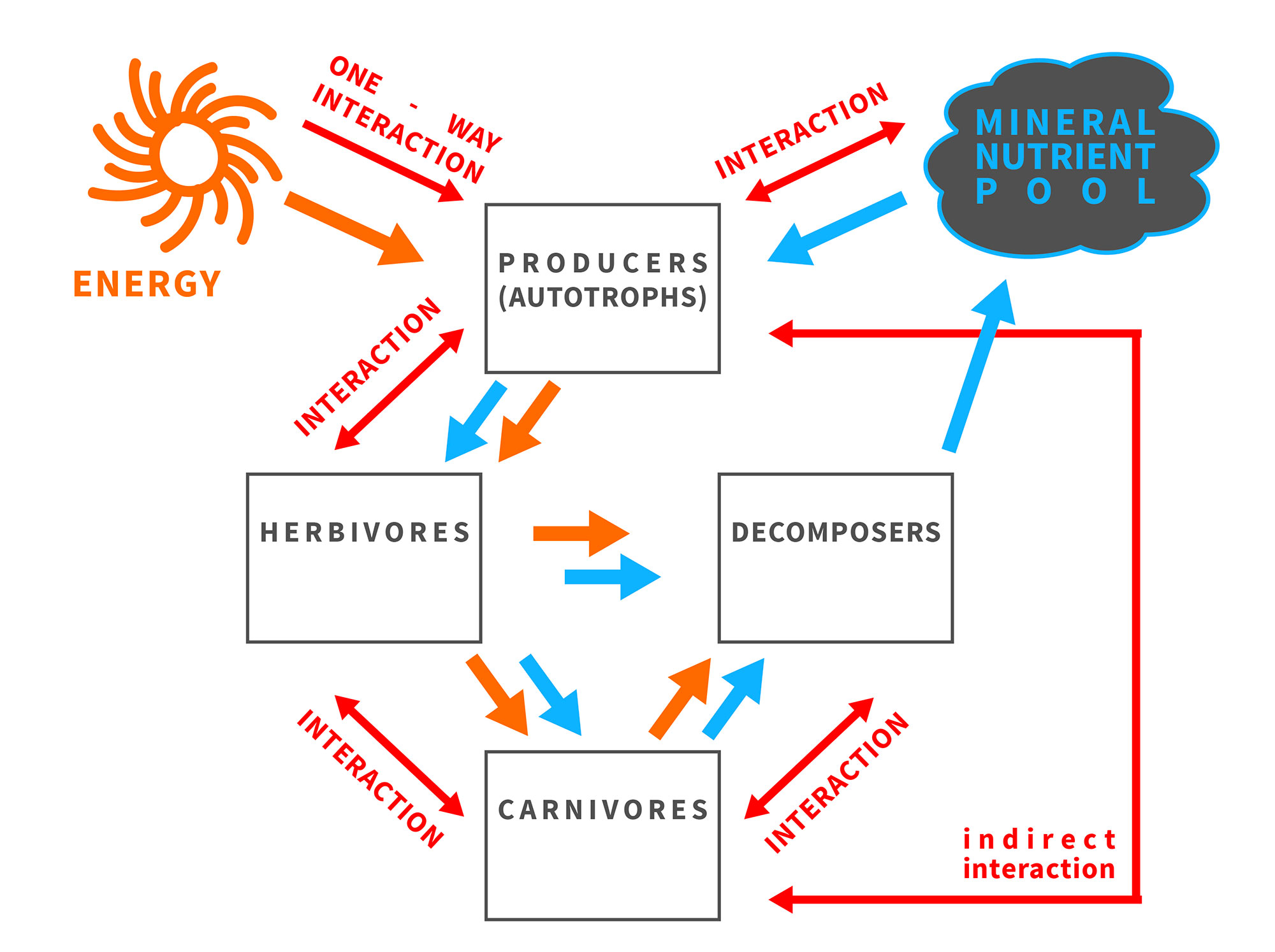
Fig. 1 The balance of energy and matter affects and is affected by organisms and their interactions in an ecosystem.

Fig. 2 Consurrent statumers that feed on nutritionally poor food (e.g. plant matter) consume a pre-packaged ratio of atoms. For herbivores the food contains more C relative to other atoms, so these organisms must manage a diet with excess C that presents a stoichiometric mismatch that limits their growth and development. The mismatch is further increased by unbalanced relation between non-C elements (exceptional scarcity of some of them).
It was noted recently that it is easier to find in the scientific literature a population's genome than its elemental composition[17]. At the same time, the concept of stoichiometric niche was introduced and defined as the region of multivariate niche space occupied by a group of individuals where the axes represent their elemental content[18][19]. If a consumer is unable to find food that fits its stoichiometric niche, a limitation will be imposed on its growth and development that negatively influences its fitness, and the entire population may be negatively impacted in this way[2][3]. In this way all consumers, especially herbivores, are faced with a high threshold of stoichiometric incompatibility between the chemical composition of their tissues and their food (Fig. 2). Ecological stoichiometry raises questions concerning the strategies of crossing this threshold (the advantages and costs involved) and how these strategies affect the structure of communities and ecosystem functioning. The strategies for crossing stoichiometric thresholds are still poorly understood.
Images were created with use of free vector graphics form www.vecteezy.com
For additional information visit this link, discussing biological stoichiometry and also this link, discussing conservation of mass.
Literature references
- Sterner RW, Elser JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press; 2002.
- Kay AD, Ashton IW, Gorokhova E, Kerkhoff AJ, Liess A, Litchman E. Toward a stoichiometric framework for evolutionary biology. Oikos. 2005;109: 6–17. doi:10.1111/j.0030-1299.2005.14048.x
- Kay AD, Vrede T. Ecological Stoichiomety: Evolutionary and Biochemical Aspects. Encycl Ecol. 2008;1–5: 1472–1481.
- Jones RH, Flynn KJ. Nutritional Status and Diet Composition Affect the Value of Diatoms as Copepod Prey. Science (80- ). American Association for the Advancement of Science; 2005;307: 1457–1459. doi:10.1126/science.1107767
- Vernadskiĭ VI. La Géochimie. Paryż: Alcan; 1924.
- Smil V. Cycles of Life: Civilization and the Biosphere. New York: Scientific American Library; 2000.
- Liebig J, Playfair LP, Webster JW. Chemistry in its application to agriculture and physiology. Cambridge,: J. Owen; [etc., etc.]; 1843. doi:10.5962/bhl.title.41248
- Lotka AJ. Elements of Physical Biology. Baltimore, Md, USA: Williams and Wilkins Company; 1925.
- Tilman D. Resource competition and community structure. Monographs in Population Biology. Princeton, NJ: Princeton University Press; 1982.
- Reiners WA. Complementary Models for Ecosystems. Am Nat. 1986;127: 59–73. doi:10.1086/284467
- Sterner RW, Hessen DO. Algal Nutrient Limitation and the Nutrition of Aquatic Herbivores. Annu Rev Ecol Syst. 1994;25: 1–29. doi:10.1146/annurev.es.25.110194.000245
- Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH. Organism Size, Life History, and N:P Stoichiometry. Bioscience. 1996;46: 674–684. doi:10.2307/1312897
- Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, et al. Nutritional constraints in terrestrial and freshwater food webs. Nature. 2000;408: 578–80. doi:10.1038/35046058
- Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow T a., Cotner JB, et al. Biological stoichiometry from genes to ecosystems. Ecol Lett. John Wiley & Sons; 2000;3: 540–550. doi:10.1046/j.1461-0248.2000.00185.x
- ASLO. ASLO honors “Algal Nutrient Limitation and the Nutrition of Aquatic Herbivores” with the 2017 John H. Martin Award. In: Association for the Sciences of Limnology and Oceanography [Internet]. 2016 p. http://aslo.org/awards/2017/sternerhessen.html. Available: http://aslo.org/awards/2017/sternerhessen.html
- Filipiak M, Weiner J. Plant–insect interactions: the role of ecological stoichiometry. Acta Agrobot. 2017;70: 1–16. doi:10.5586/aa.1710
The atoms of chemical elements build the tissues and bodies of organisms in various proportions. This fact is crucial for ecological interactions and may shape the functioning of entire ecosystems[1][20]. The most fundamental feature of the elements, enabling their use in ecological stoichiometry, is that specific atoms cannot be transformed into different atoms by the organism. This feature distinguishes atoms from organic compounds. Within this context, ecological stoichiometry provides a common currency that links the ecology of organisms to life history trade-offs and evolutionary processes entrenched in the biogeochemical economy of life[2]. This currency is the ratio of atoms that compose the bodies of organisms and their food[1].
To date, ecological stoichiometry has provided data on elemental composition patterns in different trophic levels, with non-C element body contents tending to be the lowest in autotrophs, relatively low in herbivores and the highest in predators. Stoichiometric homeostasis has been shown be weaker in autotrophs and stronger in heterotrophs[1]. Ecological stoichiometry has also revealed stoichiometric mismatches at various trophic levels and has elucidated nutrient cycling in food webs[1][20][21]. However, these studies were conducted almost exclusively in aquatic ecosystems[22][23][24][25][26]. Recent studies have researched nutrient cycling in soil ecosystems and the relationship between stoichiometry and biodiversity [27][28][29][30][31][32], and considerable efforts have been made in the field of consumer-driven nutrient recycling theory (e.g., [33][34][35][36]). Nevertheless, much still needs to be done, since only C, N and P have been studied in this respect (e.g., [37]; see [23] for a review). Notwithstanding, multielemental ecological stoichiometry approach has been used to explain the paradox of the efficient life strategy of wood eaters[38], to show the role of pollen in nutrient cycling[39], to learn about sex differences in stoichiometric phenotypes and stoichiometric niches[40], to gain new knowledge about nutritional ecology of western honey bee[41] and to develop effective strategies for wild bee conservation[42][43].
