The treatment and reduction of animal by-products has registered an increase in the awareness that this type of materials is underutilised and can represent a valuable resource if treated correctly. Consequently, it is no longer practical to dispose of animal by-products, especially when a significant amount of potential raw materials is produced, which can have a high economic potential through the production of new products with significant added value. The reuse and valorisation of animal by-products (ABPs) generated in the food retail sector can involve sending these by-products to another company/organisation or industry, where they will be processed in order to obtain added-value products. This type of valorisation originates an industrial symbiosis.
- agri-food business
- animal by-products
- food retail sector
- industrial symbiosis
- circular economy
1. Industrial Symbiosis
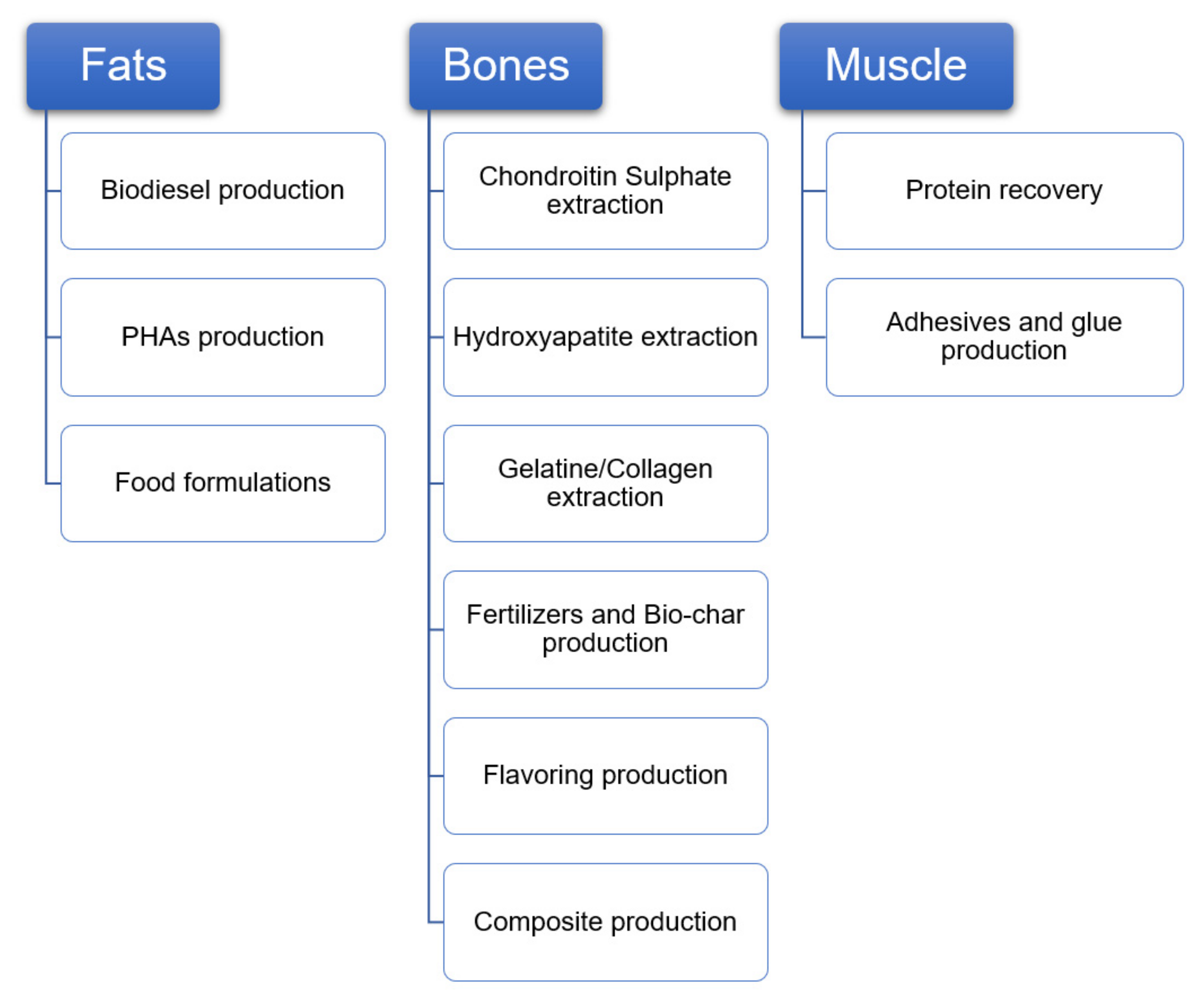
2. Implemented Industrial Processes and Technologies
| Industrial Process | Technology | Raw Materials | Final Products | Possible Uses of the Final Products | References | |
|---|---|---|---|---|---|---|
| Rendering | Digestion (133 °C; 3 bar; 20 min) for sterilisation and stabilisation of ABPs | Muscle Bones Fats |
ABP meal | Animal feed | [59,60] | [8][9] |
| Animal fat | Biodiesel production | |||||
| BioRefinex | Thermal hydrolysis (180 °C; 12 bar; 40 min) + anaerobic digestion (50–60 °C; 10–35 days) |
Muscle Bones Fats |
Hydrolysed proteins | Fertilisers | [61] | [10] |
| Biogas |
3.1. Fat Valorisation Systems
| H | 2 | O | Proteins | Lipids | Ash | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Poultry | 28.7 | 3.7 | 67.4 | 0.3 | [73] | [22] | ||||
| = 88.5% | ||||||||||
| Fuels | ||||||||||
| Bovine | [ | 76] | [25] | Gelatine production | Alkaline pre-treatment + thermal hydrolysis (50–100 °C; 10–36 h) | Bones | Gelatine powder | Food products Pharmaceuticals Photographic products | [64,65] | [13][14] |
| Chondroitin sulphate production | Enzymatic hydrolysis (60 °C; 8 h; alcalase) + alkaline hydrolysis (35 °C; 1 h; pH > 11) |
Bones | Chondroitin sulphate powder | Pharmaceuticals Cosmetics | ||||||
| Pyrolysis gas | ||||||||||
| Thermal hydrolysis (90–110 °C; 0.5–10 h) |
Muscle | Meat extract | Meat flavourings Animal feed |
[67] | [16] | |||||
| Meat powder | ||||||||||
| Enzymatic hydrolysis (50–52 °C; 50 min; papain) |
Muscle | Protein powder | Animal feed | [68] | [17] | |||||
3. Emerging Low-TRL Systems
| 4 |
| 1.5 | |||||||
| 94.0 | 0.5 | [ | 74 | ] | [23] | ||
| Rendering, mixing (methanol (6:1)), supercritical transesterification (400 °C; 41.1 MPa; 6 min) | η | biodiesel | = 88% | [77] | [26] | ||
| Beef Fat | PHA production | PHAs—polyhydroxyalkanoates Rendering, fermentation (30 °C; pH = 6.8; aeration = 0.5 vvm; C | O2 | = 40%; 300–1200 rpm) | Purity >99% Production = 0.4 g PHA/g fat Productivity = 0.36 g PHA/L.h |
[79] | [28] |
| Biodiesel production | Heating, filtration, transesterification (50 °C; KOH + methanol), decanting, washing (hot water + acetic acid), mixing (methanol), vacuum distillation, dehydration (ethylene glycol) | η | biodiesel | = 73% | [78] | [27] | |
| Pork Fat | Food formulations | Winterisation process | Decrease of 28% in the saturated fatty acid content | [80] | [29] |
3.2. Bone Valorisation Systems
| ABP | Final Use | Experimental Procedure | Results | Reference | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poultry Fat | Biodiesel production | Heating (110 °C), filtration (30 °C), transesterification (methanol (6:1) and NaOH (1%); 30 °C; 90 min), decanting (1 h), evaporation (low pressure), mixing (50% ( | v | / | v | ) HCl (0.2%)), mixing (50% ( | v | / | v | ) H | 2 | O), dehydration (Na | 2 | SO | 4 | (25%); 30 rpm), filtration | η | extraction | = 40% η | biodiesel | = 87% | [ |
| - | [ | 65 | ] | [ | 14 | ] | ||||||||||||||||
| Pig | 36.6 | 21.8 | 17.5 | 24.1 | - | [84] | [33] |
| ABP | Final Use | Experimental Procedure | Results | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Poultry Bones | Chondroitin sulphate extraction | Washing (H | 2 | O; 30 min), mixing (H | 2 | O (1.5:1)), heating (120 °C; 0.1 MPa; 120 min), filtration (100-mesh sieve), centrifugation, heat-resin static adsorption extraction, mixing (trichloroacetic acid (7% | w | / | v | ); 4°C; 24 h), centrifugation (15,000× |
| H | 2 | O | Proteins | Lipids | Ash | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | ; 20 min), mixing (ethanol (70% | v | / | v | ); 24 h), centrifugation (5000× | g | ; 5 min), drying (60 °C), mixing (H | 2 | O), ultrafiltration, freeze drying | 22.8 | η | CS | = 0.14% % recovered = 67.4% M | CS | = 35.81 kDa | 72] | [21 | |||
| 15 | ||||||||||||||||||||
| ] | ||||||||||||||||||||
| ] | ||||||||||||||||||||
| [ | 83 | ] | [ | 32] | 1.0 | 1.2 | [ | Rendering, filtration, heating (110 °C; 1 h), esterification (methanol (40%) + H | 2 | SO | 4 | (2.5%); 63 °C; 1 h), decanting, mixing (H | 2 | O; 65 °C), heating (110 °C), transesterification (methanol (20%) + KOH (1%)), decanting, mixing (H | 2 | O; 65 °C), heating (110 °C) | ||||
| Crushing, washing (acetone), filtration, drying (60 °C; 24 h), mixing (H | 2 | O (1.5:1) + trypsin), extraction (47 °C; 6 h), heating (10 min), filtration (100-mesh sieve), centrifugation (12,000× | g | ; 10 min), mixing (ethanol (70%); 4 °C; 24 h), centrifugation (5000× | g | ; 5 min), drying (60 °C) | η | biodiesel | η | CS | = 4.25% M | |||||||||
| Gelatine extraction | ||||||||||||||||||||
| Crushing (1–3 mm), washing (H | ||||||||||||||||||||
| 2 | ||||||||||||||||||||
| O), demineralisation (HCl (50 g/L); 8 °C; 2 h), washing (H | 2 | O), enzymatic treatment (neutrase; pH = 9; 50 °C), heating (100 °C), mixing (pH = 7), gelatine extraction (T; 3 h), centrifugation (30 °C; 900× | ||||||||||||||||||
| Poultry | 75.0 | 99 | ] | [ | 48] | |||||||||||||||
| CS | = 37.18 kDa | |||||||||||||||||||
| Bovine | 75.1 | 19.2 | 4.4 | 1.3 | [100] | [49] | Hydroxyapatite extraction | Washing, drying (oven), crushing, calcination (electric furnace; P | atm | ; 700 °C) | % lost mass = 28.72% | [86] | [35] | |||||||
| g | ||||||||||||||||||||
| Pig | 75.1 | 22.8 | Flavouring production | Crushing, washing (H | 2 | O (1.5:1); 10 min), hot pressure extraction (H | 2 | O; 135 °C; 120 min), filtration (200-mesh sieve), centrifugation (16,000× | g | ), evaporation (0.08–0.1 MPa; until 30% solids) | % recovery: Proteins = 83.51% Collagen = 96.81% Amino acids = 31.03–47.73% C | Ca | = 4.2–4.8 mg/g | [97] | [46] | |||||
| Gelatine and collagen extraction | Crushing (1–2 mm), mixing (H | 2 | O (1 g:2 mL)), heating (35 °C; 1 h), washing (H | 2 | O), filtration, acid treatment (HCl (1 g:2 mL); 10 °C; 24 h), washing (H | 2 | O), filtration, alkaline treatment (NaOH (1 g: 4 mL; T | room | ; 48 h), mixing (phosphoric acid until pH = 4), washing (H | 2 | O), filtration, mixing (H | 2 | O (1 g: 3 mL); 76–82 °C; 105–183 min), centrifugation (5000 g; 30 °C; 30 min), drying (oven; 42 °C) | Gel strength = 1175.8 g T | melting | = 33.71 °C T | gelling | = 25.15 °C | [88] | [37] |
| Fertiliser production | Dehydration (110 °C; 4–5 h), rendering (133 °C; 3 bar; 20 min), double calcination (electric furnace; 550 °C) |
Coal represents 24% of initial poultry meal mass Coal: 56.33% phosphate 30.7% calcium |
[94] | [43] | Pyrolysis (850 °C; 20 min) |
Bones | Bio-char | Fertilisers and adsorbents | [62] | [11] | ||||||||||
| Pig Bones | Bio-char production | Crushing (2–5 cm), precarbonisation (450 °C; N | 2 | atmosphere; 10 °C/min), crushing (0.25–0.35 mm), pyrolysis (800 °C), washing (H | 2 | O) | H = 68.3% | [95] | [44] | Bio-oil | ||||||||||
| Bovine Bones | Fuels | Bio-char production | Washing (H | 2 | O, 90 °C; 24 h), pyrolysis (350 °C; 2 h), cooling (T | room | ||||||||||||||
| ), vacuum filtration, mixing (Ca(OH) | ||||||||||||||||||||
| 2 | ||||||||||||||||||||
| until pH = 9), flocculation, centrifugation, ion exchange | H = 13.9% | Gel strength = 243.22 g µ = 4.915 cP |
[89] | [38] | ||||||||||||||||
3.3. Muscle Valorisation Systems
| ABP | Final Use | Experimental Procedure | Results | Reference | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poultry Muscle | Protein recovery (hydrolysis) | Rendering, mixing (H | 2 | O), enzymatic hydrolysis (7 h; 50 °C; pH adjustment with NaOH (5.4 M)), heating (85 °C; 15 min), centrifugation (1000× | g | ; 4 °C; 30 min), freeze drying (0.045 mbar; −44 °C) Enzymes: alcalase (pH = 8) and flavourzyme (pH = 7) |
% hydrolysed = 11.13% % recovered = 58.1% |
[101] | [50] | |||||||||||
| Sterilisation (121 °C; 15 min), enzymatic hydrolysis (phosphate buffer (50 mM); 50–56 °C; 18 h), filtration, centrifugation (15000 rpm; 30 min), filtration, spray drying (67 °C; 4 h) | Enzyme | : papain | C | proteins | = 768 mg/g C | CS | = 89.6 mg/g C | HA | = 73.9 mg/g C | amino acids | = 44.2 mg/g | [103] | [52] | |||||||
| Crushing, mixing (H | 2 | O (3:1) + NaOH), heating, enzymatic hydrolysis (52.5 °C; pH = 8 with addition of NaOH), heating (85 °C; 20 min), centrifugation (3500rpm; 20 min) | Enzyme | : alcalase (4.2%) | % hydrolysed protein = 31% % recovered protein = 91% |
[102] | [51] | |||||||||||||
| Crushing (3000 rpm; 3 min), mixing (H | 2 | O; 1100 rpm; 5 min), hydrolysis (40 °C; pH = 9; 60 min), centrifugation (10,000× | g | ; 15 min), microfiltration (2 bar), ultrafiltration (2 bar), isoelectric precipitation (HCl (37%) until pH = 4), centrifugation (5000× | g | ; 5 min), mixing (hexane + isopropanol (3:2 | v | / | v | ); 1 h; 20 °C), evaporation | η | extraction | = 83% η | process | = 55% | [107] | [56] | |||
| Crushing (2.3 mm), ISP (H | 2 | O + TiO | 2 | (6:1); 32–34 °C), mixing (NaOH until pH = 11.5; 10 min), centrifugation (10,000× | g | ; 10 min), mixing (HCl until pH = 5.5; 10 min), centrifugation | Addition of TiO | 2 | to the ISP-recovered proteins resulted in increased gel strength | [108] | [57] | |||||||||
| ), crushing (75–300 µm) | ||||||||||||||||||||
| Adsorption capacity = | 10.56 mg F/g | [ | 92] | [41] | ||||||||||||||||
| Washing, drying (110 °C), crushing (1–2 mm), washing (acetone + H | 2 | |||||||||||||||||||
| Mixing (H | 2 | O; pH = 4–4.5; 3.5 h), filtration, centrifugation, evaporation (until 25° Brix), enzymatic hydrolysis (aspergillus (0.06%); 50 °C; pH = 7; 1 h), heating (10 min), enzymatic hydrolysis (trypsin/chymotrypsin (1%); 37 °C; pH = 7; 1 h), heating (10 min), centrifugation |
Inhibitory activity: Chicken extract = 1060 mg% Hydrolysed extract = 1.1 mg% |
[104] | [53] | |||||||||||||||
| Adhesive and glue production | Mixing (H | 2 | O (1:4 | w | / | v | ); 10 min), filtration (200-mesh sieve), centrifugation (10,000× | g | ; 4 °C; 25 min), mixing (NaOH (2 M) until pH = 11), centrifugation (10,000× | g | ; 4 °C; 25 min), mixing (HCl (2 M) until pH = 5), centrifugation, washing (H | 2 | O), freeze drying, mixing (sodium dodecyl sulphate (3 M) or urea (3%) and NaOH (10%) until pH = 10) | Urea (3%)/SDS (3 M): Dry strength = 7.99/9.35 MPa Wet strength = 3.35/2.9 MPa Soaked strength = 5.21/8.89 MPa |
[109] | [58] | O), filtration, drying, pyrolysis (400 °C; 2 h; 10.2 °C/min) | Removal of 41.4% of 17-β oestradiol from water | [93] | [42] |
| Composite production | Crushing, washing (H | 2 | O), drying, carbonisation (550 °C; 1 h), crushing (100 µm) | Composite strength increases with bone carbonisation | [96] | [45] | ||||||||||||||
| Hydroxyapatite extraction | Washing (H | 2 | O; 1 h), washing (acetone; 2 h), drying, crushing (45–125 µm), calcination (T; 3 h; 10 °C/min) | Optimal calcination temperature ≥700 °C |
[85] | [34] | ||||||||||||||
References
- Chertow, M.R. Industrial symbiosis: Literature and Taxonomy. Annu. Rev. Energy Environ. 2000, 25, 313–337.
- Lombardi, D.R.; Laybourn, P. Redefining Industrial Symbiosis. J. Ind. Ecol. 2012, 16, 28–37.
- Chertow, M.R. Uncovering’ Industrial Symbiosis. J. Ind. Ecol. 2007, 11, 11–30.
- Fraccascia, L.; Magno, M.; Albino, V. Business models for industrial symbiosis: A guide for firms. Procedia Environ. Sci. Eng. Manag. 2016, 3, 83–93.
- Neves, A.; Godina, R.; Azevedo, S.G.; Matias, J.C.O. A comprehensive review of industrial symbiosis. J. Clean. Prod. 2020, 247, 119113.
- Lowe, E.A. Creating by-product resource exchanges: Strategies for eco-industrial parks. J. Clean. Prod. 1997, 5, 57–65.
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives. Polymers 2018, 10, 176.
- Meeker, D.L. Essential Rendering—All About THE Animal By-Products Industry; National Renderers Association: Alexandria, VA, USA, 2006.
- Campos, I.; Valente, L.M.P.; Matos, E.; Marques, P.; Freire, F. Life-cycle assessment of animal feed ingredients: Poultry fat, poultry by-product meal and hydrolyzed feather meal. J. Clean. Prod. 2020, 252, 119845.
- Facey, R.M.; Stavne, A. Apparatus and Process for Production of Biogas. Patent CA 2641270, 25 December 2009.
- Someus, E.; Pugliese, M. Concentrated Phosphorus Recovery from Food Grade Animal Bones. Sustainability 2018, 10, 2349.
- European Commission. Communication on the 2017 List of Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2017; pp. 1–8.
- Schrieber, R.; Gareis, H. Gelatine Handbook—Theory and Industrial Practice; Wiley: Hoboken, NJ, USA, 2007.
- European Commission. Best Available Techniques in the Slaughterhouses and Animal By-Products Industries; European IPPC Bureau: Seville, Spain, 2005.
- Chomarat, F.N.; Grech, G.P.; Cueto, L.G.; Rodriguez, E.; Boe, J.-F. Method for the Preparation of Sodium Chondroitin Sulphate. U.S. Patent 2013/0172289 A1, 4 July 2013.
- Meat and Livestock Australia, “Edible Meat Powders and Extracts.” 2001. Available online: https://meatupdate.csiro.au/infosheets/Edible%20Meat%20Powders%20and%20Extracts.pdf (accessed on 20 February 2021).
- Scheide-Fisher, I.; Scheide, J.D. Method of Manufacturing Meat Extract. U.S. Patent 2011/0250316 A1, 13 October 2011.
- Toldrá, F.; Reig, M.; Mora, L. Management of meat by- and co-products for an improved meat processing sustainability. Meat Sci. 2021, 181, 108608.
- Mosna, D.; Bottani, E.; Vignali, G.; Montanari, R. Environmental benefits of pet food obtained as a result of the valorisation of meat fraction derived from packaged food waste. Waste Manag. 2021, 125, 132–144.
- Liu, N.; Jiang, J. Valorisation of food waste using salt to alleviate inhibition by animal fats and vegetable oils during anaerobic digestion. Biomass-Bioenergy 2020, 143, 105826.
- Moreira, A.L.; Dias, J.M.; Almeida, M.F.; Alvim-Ferraz, M.C.M.; Alvim-Ferraz, M.D.C. Biodiesel Production through Transesterification of Poultry Fat at 30 °C. Energy Fuels 2010, 24, 5717–5721.
- U.S. Department of Agriculture. Chicken, Broilers or Fryers, Separable Fat, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171468/nutrients (accessed on 3 July 2020).
- U.S. Department of Agriculture. Beef, Retail Cuts, Separable Fat, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168605/nutrients (accessed on 3 July 2020).
- Food and Agriculture Organization of the United Nations. “Composition of Meat”. 2020. Available online: http://www.fao.org/ag/againfo/themes/en/meat/backgr_composition.html (accessed on 8 July 2020).
- Emiroğlu, A.O.; Keskin, A.; Şen, M. Experimental investigation of the effects of turkey rendering fat biodiesel on combustion, performance and exhaust emissions of a diesel engine. Fuel 2018, 216, 266–273.
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J. Supercrit. Fluids 2010, 54, 53–60.
- Souissi, Y.; Alouini, M.; Mnif, W. Chemical and Biological Investigation of Organic Wastes of Frying Oils and Beef Fats: Valorization for Biodiesel Production. J. Chem. 2018, 2018, 6248047.
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J. Biotechnol. 2015, 214, 119–127.
- Amorim, C.D.; Camilo, A.G.; De Oliveira, C.; Petenucci, M.E.; Fonseca, G.G. Turning pork processing waste into value-added chemicals for the food industry. Sustain. Mater. Technol. 2015, 6, 1–5.
- Shirsath, A.P.; Henchion, M.M. Bovine and ovine meat co-products valorisation opportunities: A systematic literature review. Trends Food Sci. Technol. 2021, 118, 57–70.
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.; Ramadass, K.; Vinu, A.; Kirkham, M.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2020, 272, 115985.
- Wang, X.; Shen, Q.; Zhang, C.; Jia, W.; Han, L.; Yu, Q. Chicken leg bone as a source of chondroitin sulfate. Carbohydr. Polym. 2018, 207, 191–199.
- Field, R.A.; Riley, M.L.; Meuo, F.C.; Corbfidge, M.H. Bone composition in cattle, pigs, sheep and poulty. J. Anim. Sci. 1974, 39, 493–499.
- Khoo, W.; Nor, F.; Ardhyananta, H.; Kurniawan, D. Preparation of Natural Hydroxyapatite from Bovine Femur Bones Using Calcination at Various Temperatures. Procedia Manuf. 2015, 2, 196–201.
- Bee, S.-L.; Mariatti, M.; Ahmad, N.; Yahaya, B.; Hamid, Z.A. Effect of the calcination temperature on the properties of natural hydroxyapatite derived from chicken bone wastes. Mater. Today Proc. 2019, 16, 1876–1885.
- Azzallou, R.; Ouerghi, O.; Geesi, M.H.; Riadi, Y.; Taleb, M.A.; Mamouni, R.; Lazar, S.; Kaiba, A.; Kamal, M.; Villain, S. Bovine bone-derived natural hydroxyapatite-supported ZnCl2 as a sustainable high efficiency heterogeneous biocatalyst for synthesizing amidoalkyl naphthols. J. Phys. Chem. Solids 2022, 163, 110533.
- Erge, A.; Zorba, Ö. Optimization of gelatin extraction from chicken mechanically deboned meat residue using alkaline pre-treatment. LWT 2018, 97, 205–212.
- Hosseini-Parvar, S.H.; Keramat, J.; Kadivar, M.; Khanipour, E.; Motamedzadegan, A. Optimising conditions for enzymatic extraction of edible gelatin from the cattle bones using response surface methodology. Int. J. Food Sci. Technol. 2009, 44, 467–475.
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198.
- Pramualkijja, T.; Pirak, T.; Euston, S.R. Valorization of chicken slaughterhouse by-products: Production and properties of chicken trachea hydrolysates using commercial proteases. Int. J. Food Prop. 2021, 24, 1642–1657.
- Shahid, M.K.; Kim, J.Y.; Choi, Y. Synthesis of bone char from cattle bones and its application for fluoride removal from the contaminated water. Groundw. Sustain. Dev. 2019, 8, 324–331.
- Patel, S.; Han, J.; Qiu, W.; Gao, W. Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones, for environmental application. J. Environ. Chem. Eng. 2015, 3, 2368–2377.
- Deydier, E.; Guilet, R.; Sarda, S.; Sharrock, P. Physical and chemical characterisation of crude meat and bone meal combustion residue: “Waste or raw material?”. J. Hazard. Mater. 2005, 121, 141–148.
- Iriarte-Velasco, U.; Sierra, I.; Zudaire, L.; Ayastuy, J.L. Preparation of a porous biochar from the acid activation of pork bones. Food Bioprod. Process. 2016, 98, 341–353.
- Harish, S.; Guptha, N.V. The study of tensile and flexural strength of cattle bone particulate reinforced epoxy. Mater. Today Proc. 2018, 5, 20927–20931.
- Wang, J.; Dong, X.; Yue, J.; Zhang, C.; Jia, W.; Li, X. Preparation of Substrate for Flavorant from Chicken Bone Residue with Hot-Pressure Process. J. Food Sci. 2016, 81, C578–C586.
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239.
- Besler, M.; Fiocch, A.; Restani, P. Allergen Data Collection: Chicken Meat (Gallus domesticus). Internet Symp. Food Allerg. 2001, 3, 193–201.
- Besler, M.; Fiocch, A.; Restani, P. Allergen Data Collection: Beef (Bos domesticus). Internet Symp. Food Allerg. 2001, 3, 171–184.
- Nchienzia, H.; Morawicki, R.; Gadang, V. Enzymatic hydrolysis of poultry meal with endo- and exopeptidases. Poult. Sci. 2010, 89, 2273–2280.
- Kurozawa, L.; Park, K.J.; Hubinger, M. Optimization of the Enzymatic Hydrolysis of Chicken Meat Using Response Surface Methodology. J. Food Sci. 2008, 73, C405–C412.
- Stiborova, H.; Kronusova, O.; Kastanek, P.; Brazdova, L.; Lovecka, P.; Jiru, M.; Belkova, B.; Poustka, J.; Stranska, M.; Hajslova, J.; et al. Waste products from the poultry industry: A source of high-value dietary supplements. J. Chem. Technol. Biotechnol. 2020, 95, 985–992.
- Saiga, A.; Okumura, T.; Makihara, T.; Katsuta, S.; Shimizu, T.; Yamada, R.; Nishimura, T. Angiotensin I-Converting Enzyme Inhibitory Peptides in a Hydrolyzed Chicken Breast Muscle Extract. J. Agric. Food Chem. 2003, 51, 1741–1745.
- Wang, D.; Shahidi, F. Protein hydrolysate from turkey meat and optimization of its antioxidant potential by response surface methodology. Poult. Sci. 2018, 97, 1824–1831.
- Thoresen, P.P.; Álvarez, R.G.; Vaka, M.R.; Rustad, T.; Sone, I.; Fernández, E.N. Potential of innovative pre-treatment technologies for the revalorisation of residual materials from the chicken industry through enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2020, 64, 102377.
- Selmane, D.; Christophe, V.; Gholamreza, D. Extraction of proteins from slaughterhouse by-products: Influence of operating conditions on functional properties. Meat Sci. 2008, 79, 640–647.
- Tahergorabi, R.; Sivanandan, L.; Jaczynski, J. Dynamic rheology and endothermic transitions of proteins recovered from chicken-meat processing by-products using isoelectric solubilization/precipitation and addition of TiO2. LWT 2012, 46, 148–155.
- Wang, C.; Wu, J. Preparation and characterization of adhesive from spent hen proteins. Int. J. Adhes. Adhes. 2012, 36, 8–14.
- Toldrá, F.; Mora, L.; Reig, M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59.
- European Commission. Regulation (EC) No 1069/2009 Laying Down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002—Animal by-products Regulation. Off. J. Eur. Union 2009, 300, 1–33.
- Lifevalporc. Valorization of Pig Carcasses through Their Transformation into Biofuels and Organic Fertilizers. 2017. Available online: http://lifevalporc.eu/Lifevalporc (accessed on 21 October 2020).
