Healthy diets prevent diet-related non-communicable diseases; they provide nutrients and health-promoting substances from nutritious foods in adequate amounts. With society's growing interest in healthy eating, the interest in fresh, ready-to-eat, functional food, such as microscale vegetables (sprouted seeds and microgreens), has been on the rise in recent years globally. This entry briefly describes the crops commonly used for microscale vegetable production, highlights Brassica vegetables because of their health-promoting secondary metabolites and looks at consumer acceptance of sprouts and microgreens. Landraces, wild food plants, and crops' wild relatives often have high phytonutrient density and exciting flavours and tastes, thus providing scope to widen the range of crops and species used for this purpose. Moreover, the nutritional value and content of phytochemicals often vary with plant growth and development stages of the same crop. Sprouted seeds and microgreens are often more nutrient-dense than ungerminated seeds or mature vegetables. This entry also describes the environmental and priming factors that may impact the nutritional value and content of phytochemicals of microscale vegetables. Due to their short growth cycle, nutrient-dense sprouts and microgreens can be produced with minimal input and without pesticides. They can even be home-grown and harvested as needed, hence having low environmental impacts and a broad acceptance among health-conscious consumers.
- microscale vegetables
- sprouts
- microgreens
- phytonutrients
- functional foods
- seed priming
- biofortification
- illumination
1. Introduction
2. Crops Commonly Used for Microscale Vegetable Production
Sprouts and microgreens are grown from the seeds of many crops, such as legumes, cereals, pseudo-cereals, oilseeds, vegetables, and herbs [10][25]. The significant traits of interest for consumers are the appearance, texture, flavor, phytochemical composition, and nutritional value of sprouts and microgreens [26]. Most crops are grown for sprouts and microgreens, except for beans and some oilseed tree species that are commonly grown as sprouts only. Mungbean and soybean sprouts have long been an essential, year-round component of Asian and vegetarian dishes [10][27]. Mungbean sprouts have become increasingly popular in the Americas, Europe, and Africa. They are commonly recognized as “bean sprouts,” although this group comprises several different crops. Most food and forage legumes are known for their high nutritional value and an abundance of minerals and secondary metabolites. Sprouted seeds and microgreens often contain higher concentrations of bioactive compounds than raw seeds (Figure 1, Figure 2 and Figure 3) [28][29]. Sprouting cereal grains enhances their nutritional value, especially when applying a sprouting duration of at least 3 to 5 days [30]. The sprouting process activates hydrolytic enzymes and releases nutrients from their phytate chelates, making them bioavailable; in addition, vitamins are synthesized and accumulate [30]. Sprouted grains are also used in many staple foods such as bread, pasta, noodles, and breakfast flakes, but food processing often compromises their nutritional value. Pseudocereals are underutilized food crops that are receiving increasing attention as highly nutritious and functional foods [31]. Among those, amaranth, quinoa, and buckwheat are increasingly becoming popular for sprout and microgreen production [32][33][34][35]. Apart from soybean, peanut (listed under legumes), and mustard (classified here as a vegetable), almond, hazelnut, linseed, sesame, and sunflower are other oilseed crops that one can use for sprouting or microgreen production. Among the group of vegetables and herbs, members of the Brassicaceae family are widely used for sprouting and microgreen production, followed by crops of the Apiaceae, Fabaceae, and Amaranthaceae families.

-
Seed activation through imbibition, favourable temperature, oxygen, light, or darkness
-
Enhanced respiration and metabolic activities
-
Enzymes mobilize stored seed reserves and convert starch to sugar
-
Hydrolysis of storage proteins, release of essential amino acids
-
Accumulation of phenolic compounds with antioxidant ability
-
Accumulation of vitamins (C, folate, thiamin, pyridoxin, tocopherols, niacin, etc.).
-
Phytate, oxalate, and tannin degradation, leading to enhanced palatability, improved bioaccessibility of iron and calcium and enhanced digestibility of proteins.

-
Photosynthetic activity in microgreens further enhances vitamin C, phylloquinone, and tocopherol accumulation compared to sprouts
-
Accumulation of carotenoids is often higher than in mature vegetables
-
Increased accumulation of chlorophyll and phenolic compounds with antioxidant ability, compared to sprouts
-
Often higher content of macro- and micronutrients and lower content of nitrate in microgreens compared to the adult growth stage
-
Biofortification with specific elements (iodine, iron, zinc, selenium) made easy in hydroponic systems
-
Microgreens are consumed raw, hence thermolabile ascorbic acid content can be fully utilized, unlike in cooked mature vegetables.
2.1. Bioactive Composition and Potential Health Effects of Brassica Microscale Vegetables
The Brassicaceae family comprises a wide range of crops commonly used for microscale vegetable production. The intrinsic qualities of Brassica vegetables, including their color, aroma, taste, and health properties, are profoundly determined by secondary plant metabolite profiles and their concentrations in plant tissues [24]. Brassica vegetables are rich sources of bioactive compounds, such as glucosinolates (GSLs), polyphenols, anthocyanins, ascorbic acid, carotenoids, and tocopherols [36][37][38][39]. The biosynthesis of secondary plant metabolites is closely linked to plant protection and defense mechanisms and can be modulated by environmental and agronomical factors. Those factors may significantly change the concentration of secondary plant metabolites with up to 570-fold increases for specific compounds, such as isothiocyanate [24]. Among the bioactive compounds of Brassica vegetables, polyphenols and GSLs have been widely studied due to their known health-promoting effects [40][41], including the impact of cooking methods on the retention of these essential compounds [42]. In addition, polyphenols are good sources of natural antioxidants, which help decrease the risk of diseases associated with oxidative stress [43]. GSLs, defined as aliphatic, aromatic, or indolic based on their side chains, are important secondary metabolites that are predominantly found in Brassica crops [44]. In vitro studies have demonstrated the cancer-preventive potential of kale (B. carinata) has been demonstrated through in vitro studies which indicated the protection of human liver cells against aflatoxin in vitro [45]. Rose et al. [46] obtained similar results with broccoli (Brassica oleracea var. italica) and watercress (Nasturtium officinale). Isothiocyanates—hydrolysis products of GSLs—extracted from broccoli and watercress sprouts suppressed human MDA-MB-231 breast cancer cells in vitro. In addition, extracts of 3-day-old broccoli sprouts were highly effective in reducing the incidence, multiplicity, and rate of development of mammary tumors in rats treated with the carcinogen DMBA (7,12-dimethylbenz[a]anthracene) [47]. Therefore, diets high in Brassica vegetables may contribute to the suppression of carcinogenesis, and this effect is at least partly related to their relatively high content of GSLs [48]. Among five of the microgreen species of the Brassicaceae, namely broccoli (Brassica oleracea var. italica), daikon (Raphanus raphanistrum subsp. sativus), mustard (Brassica juncea), rocket salad (Eruca vesicaria), and watercress (Nasturtium officinale), broccoli had the highest polyphenol, carotenoid, and chlorophyll contents, as well as strong antioxidant power [39]. Mustard microgreens showed high ascorbic acid and total sugar contents. On the other hand, rocket salad exhibited the lowest antioxidant content and activity among the five evaluated microgreen crops [39]. Broccoli, curly kale, red mustard, and radish microgreens are good sources of minerals. They provide considerable amounts of vitamin C (31–56 mg/100 g fresh weight) and total carotenoids (162–224 mg β-carotene/100 g dry weight), the latter being higher than in adult plants [49]. In digestion studies, total soluble polyphenols and total isothiocyanates showed a bioaccessibility of 43–70% and 31–63%, respectively, while the bioaccessibility of macroelements ranged from 34–90% [49]. Among the four microgreen crops tested, radish and mustard presented the highest bioaccessibility of bioactive compounds and minerals.2.2. Consumer Acceptance of Sprouts and Microgreens and Nutritional Profile of Microscale Vegetables
Six commonly grown and consumed microgreen species were tested by Michell et al. [50] for consumer acceptance, as follows: (a) Brassicaceae: arugula (Eruca sativa), broccoli (Brassica oleracea var. italica), and red cabbage (B. oleracea var. capitata); (b) Amaranthaceae: bull’s blood beet (Beta vulgaris) and red garnet amaranth (Amaranthus tricolor); and (c) Fabaceae: tendril pea (Pisum sativum). All six microgreen crops received high ratings for appearance acceptability; hence they could easily be used to enhance the visual appearance of meals if they have the appropriate sensory attributes [50]. Among the six microgreen crops evaluated, broccoli, red cabbage, and tendril pea received the highest overall acceptability score with similar trends for taste and texture. In a similar approach, Xiao et al. [26] evaluated six microgreen species for their sensory attributes and nutritional value. The six species consisted of (i) three Brassicaceae crops: Dijon mustard—Brassica juncea, peppercress—Lepidium bonariense, and China rose radish—Raphanus sativus; (ii) two representatives of the Amaranthaceae family: bull’s blood beet—Beta vulgaris and red amaranth—Amaranthus tricolor; and (iii) one representative of the Lamiaceae family: opal basil—Ocimum basilicum. Overall, all six microgreen species received “good” to “excellent” consumer acceptance ratings and showed high nutritional quality. Among those six crops, bull’s blood beet received the highest acceptability score regarding flavor and overall eating quality, while peppercress received the lowest score [26]. In addition, the authors detected the highest concentrations of total ascorbic acid and tocopherols in China rose radish, the highest contents of total phenolics and phylloquinone (vitamin K1) in opal basil, and the highest content of carotenoids in red amaranth. In trials conducted at the World Vegetable Center, the consumer acceptance of amaranth (Amaranthus tricolor) landraces, conserved in the Genebank, were compared with commercially available cultivars [32]. A Genebank accession (VI044470) consistently received the highest ratings for appearance, texture, taste, and general acceptability at the sprout, microgreen, and fully grown stages.A consumer acceptance study conducted in India comprised the following ten microgreens: carrot, fenugreek, mustard, onion, radish, red roselle, spinach, sunflower, fennel, and French basil [51]. The organoleptic acceptability of all ten microscale vegetables ranged from very good to excellent.
The high appreciation of microgreens compared to mature vegetables might also be related to their aroma profile. Recent research undertaken by Dimita et al. [52] has shown that the aroma profile of Perilla frutescens var. frutescens (Chinese basil or perilla; green leaves) and P. frutescens var. crispa (red leaves) is much higher at the microgreens stage than at the later adult stage. Both varieties have a clearly distinct aroma profile at the microgrreens stage. The red variety emitted a citrusy, spicy, and woody aroma, while the green type produced a fruity, sweet, spicy, and herbaceous aroma at the microgreens stage [52]. After the microgreen stage, at the age of four weeks, green Chinese basil no longer emitted any aroma volatiles. Hence, the aroma profile of Chinese basil leaves at the microgreen stage is clearly variety-specific and not related to the content of total phenols or the antioxidant capacity of the leaves. Attempting a nutritional determination among five Brassicaceae microgreen crops (broccoli, daikon, mustard, rocket salad, and watercress), broccoli excelled [39]. Broccoli microgreens had the highest content of isothiocyanates, known for their cancer-preventing abilities [47][48] and displayed the most potent antioxidant power. Broccoli microgreens exhibited the overall best nutritional profile and, therefore, are considered as one of the most promising functional food species [39]. Based on the determination of the contents of 11 nutrients and vitamins, as well as the anti-nutrient oxalic acid, and their relative contribution to the diet as per the estimated daily intake published in the United States Department of Agriculture (USDA) database for green leafy vegetables, Ghoora et al. [53] computed a nutrient quality score (NQS) to assess the nutritional quality of ten culinary microgreen species. The selected species included vegetable crops (spinach, carrot, mustard, radish, roselle, and onion); leguminous crops (fenugreek); oleaginous crops (sunflower); and aromatic species (French basil and fennel). All microgreen crops are moderate to good sources of protein, dietary fiber, and essential nutrients. Concerning their vitamin content, the studied microgreens are excellent sources of ascorbic acid, vitamin E, and beta-carotene (pro-vitamin A), meeting 28–116%, 28–332%, and 24–72% of reference daily intake of the respective vitamins [53]. In general, microgreens had low levels of oxalic acid, which is a predominant anti-nutrient in mature leafy vegetables. Based on the calculated NQS, radish microgreens showed the highest nutrient density, followed by French basil and roselle microgreens. On the other hand, fenugreek and onion microgreens are the least nutrient-dense. Furthermore, the calculated NQS revealed that all microgreens were 2–3.5 times more nutrient-dense than mature leaves of spinach cultivated under similar conditions. While high nutrient density and high phytochemical content are considered a must in sprouts and microgreens, these microscale vegetables must also have high consumer acceptability in flavor attributes and visual appearance. Based on organoleptic and nutritional properties, Caracciolo et al. [54] assessed different microgreens species regarding consumer acceptance of appearance, texture, and flavor. The 12 microgreen species included in the studies were amaranth, coriander, cress, green basil, komatsuna, mibuna, mizuna, pak choi, purple basil, purslane, Swiss chard, and tatsoi. The results revealed that while the visual appearance of the microgreens played a role, the flavor and texture of microgreens were the main determining factors for consumer acceptance. In general, low astringency, sourness, and bitterness enhanced the consumer acceptability of microgreens [54]. Among the 12 examined microgreen species, mibuna (Brassica rapa subsp. nipposinica) and cress (Lepidium sativum) received the lowest consumer acceptance score, while Swiss chard (Beta vulgaris subsp. vulgaris) and coriander (Coriandrum sativum) were the most appreciated microscale vegetables. Unfortunately, phenolic content strongly correlates with flavor attributes such as sourness, astringency, and bitterness. Therefore, microscale vegetables rich in phenolics, such as red cabbage (Brassica oleracea var. capitata), sorrel (Rumex acetosa), and peppercress (Lepidium bonariense), in general, receive a low consumer acceptability score [11][26]. However, rich content in minerals, vitamins, phenolics, and antioxidant activity can also be found in species of more acceptable tastes, such as amaranth, coriander, and Swiss chard [11][14][55][32][56][57]. As shown with the above examples, identifying microgreen species that satisfy both sensory and health attributes at a high degree remains a challenge since acrid taste’s acceptability is subject to both inherited and acquired taste factors [11]. Providing concise, crop-specific information about the culinary uses and the outstanding nutritional and health benefits of microscale vegetables might increase consumer interest. Such information might convince them to try products of high nutritional value but less agreeable tastes, eventually broadening the overall consumer acceptability of such produce [54].3. Underutilized Species with Potential for Microscale Vegetable Production to Enhance Nutrition Security
Breeding for high yield, appearance, etc., may sometimes unintentionally lead to a decline in essential nutrients and phytochemicals. This hypothesis is supported by a review study conducted on 43 garden crops that revealed a statistically reliable reduction in six nutritional factors (protein, Ca, P, Fe, riboflavin, and ascorbic acid) between 1950 and 1999 based on USDA food composition data for this period [58]. These changes can be explained by changes in the crop varieties cultivated during this same period. Similar trends have been observed in wheat grain [59][60] and potato tubers [6[1]. Marles [61] conceded that some modern varieties of vegetables and grains might have lower contents in some nutrients than older varieties due to a dilution effect of increased yield by the accumulation of carbohydrates without a proportional increase in certain other nutrients. Nevertheless, he argued that eating the WHO-recommended daily servings of fruits and vegetables would provide adequate nutrition [61]. Nonetheless, it is known that most countries and the majority of the global population, especially in sub-Saharan Africa, are still well below the WHO-recommended daily intake levels of fruits and vegetables. When aiming for high phytonutrient density and exciting flavors and tastes, it might well be worth exploring farmers’ landraces, wild food plants, or populations found in a semi-wild or wild state, such as crops’ wild relatives. Such species are often part of the conservation focus of national genebanks, e.g., the genebanks maintained by the USDA in the USA, or international ones, e.g., the Genebank maintained by the World Vegetable Center (WorldVeg). This idea of exploring landraces, wild food plants, or crops’ wild relatives for microscale vegetable production has recently gained impetus [11][15][21][62][51]. The microgreens of wild plants and culinary herbs could constitute a source of functional food with attractive aromas, textures, and visual appeal, which could provide health benefits due to their elevated nutraceutical value and could be exploited in new gastronomic trends [14][25][26]. Studies of 13 wild edible plants from 11 families undertaken by Romojaro et al. [63] revealed that their outstanding nutritional value would merit promotion to provide health benefits. Fennel, which is commonly used for sprout and microgreen production, has higher radical scavenging activity, total phenolic, and total flavonoid contents in its wild form compared to medicinal and edible fennel [64]. Variations in the phytochemical content of wild fennel obtained from different geographical areas were also reported. For broccoli, kale, and pak choi, there is a variation of the concentrations of secondary plant metabolites among cultivars with ranges up to 10-fold [24]. Studies involving three wild leafy species, Sanguisorba minor (salad burnet), Sinapis arvensis (wild mustard), and Taraxacum officinale (common dandelion), at the microgreen and baby green stages, were conducted by Lenzi et al. [21]. The authors recognized the potential of those wild edible plants in achieving competitive yields and contributing to the dietary intake of nutritionally essential macro- and microelements, as well as bioactive compounds. Sprouted seeds of chia (Salvia hispanica), golden flax, evening primrose, phacelia and fenugreek are an excellent source of health-promoting phytochemicals, especially antioxidants and minerals [65]. Germination significantly increased the total phenolic content (e.g., from 1.40 to 4.54 mg GAE g−1 in fenugreek and from 0.33 to 5.88 mg GAE g−1 in phacelia), antioxidant activity (e.g., 1.5 to 52.5-fold in fenugreek and phacelia, respectively), and the content of phenolic acids and flavonoids in sprouts compared to the ungerminated seed of the mentioned species. A rather exotic medicinal vegetable with a mild, bitter flavor is Korean ginseng (Panax ginseng). Sprouts of this crop can be grown to whole plants in 20 to 25 days in a soil-less cultivation system [66][67]. Their main bioactive compounds are ginsenosides which have anti-cancer, anti-diabetic, immunomodulatory, neuroprotective, radioprotective, anti-amnestic, and anti-stress properties (see references in [66]). Korean ginseng sprouts can be included in salads, milkshakes, sushi, soups, and tea. Korean ginseng is also used in health food supplements and cosmetics. In summary, underutilized plants, such as farmers’ landraces, wild food plants, or crops’ wild relatives, often conserved in genebanks, might offer valuable opportunities to produce sprouts and microgreens with high nutritional value and exciting flavors and tastes, thus meeting the demands of health-conscious consumers. However, additional research efforts are required to determine whether the germination performance of these novel plant materials is satisfactory for commercial microscale vegetable production.4. Variation of Nutritional Value and Content of Phytochemicals According to Plant Growth Stages
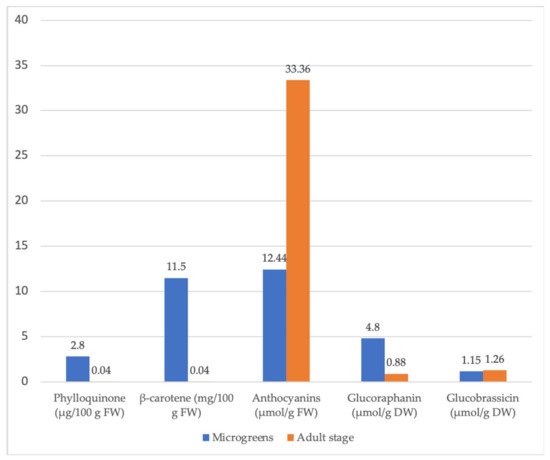
Numerous studies have shown that the nutritional value and content of phytochemicals of vegetables and other crops may vary with plant growth and development. The concentration of essential minerals, vitamins, bioactive compounds, and antioxidant activity often increases in this sequence: raw seeds—sprouted seeds—microgreens (Figure 4) [28]. In many cases, sprouts and microgreens even exceed the nutritional value of fully grown plants (Figure 5) [68].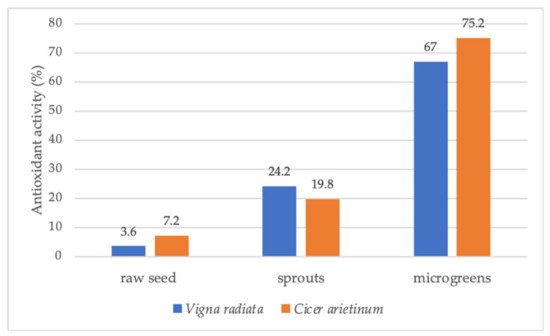
5. Environmental and Priming Factors That Have an Impact on the Nutrient and Phytochemical Content of Sprouts and Microgreens
Many factors determine the contents of nutrients and phytochemicals in microscale vegetables, such as the selected crop and cultivar, the chosen genotype’s breeding status, and the growth stage. Other factors that may impact the nutritional quality of microscale vegetables are the environment in which they are grown, the selected illumination, substrates used, nutrient biofortification, and salinity stress. On the other hand, packaging methods and storage temperature help retain nutrients and phytochemicals [55]. All these factors may influence microscale vegetables’ photosynthetic and metabolic activities and may improve nutritional quality, depending on the crop/species and genotype used.5.1. The Effect of Growth Environment and Growing Substrates
5.2. Response to Environmental Stresses
Polyphenols play a fundamental role in the defense system of plants against heavy metals, salinity, drought, extreme temperatures, pesticides, and ultraviolet (UV) radiations [70]. In response to environmental stresses, plants produce diverse metabolites, which also contribute to the functional quality of edible plant parts, such as mineral nutrients, amino acids, peptides, proteins, vitamins, pigments, and other primary and secondary metabolites [71]. The application of eustress, i.e., mild to moderate salinity or nutritional stress, can elicit targeted plant responses by activating physiological and biochemical mechanisms. These, in turn, may lead to the accumulation of desired bioactive compounds in the harvested produce [72]. Salinity eustress may enhance health-promoting phytochemicals such as lycopene, β-carotene, vitamin C, and polyphenols in vegetables [72]. For example, exposing Se-biofortified maize grains to mild NaCl stress (i.e., 25 mM NaCl) during germination resulted in good sprout yields, increased the content of selenocysteine, and boosted the synthesis of pro-nutritional semipolar metabolites with antioxidant properties [73]. Nutrient deprivation in wild rocket (Diplotaxis tenuifolia) microgreen production elicited a substantial increase in secondary metabolites, such as lutein (110%), β-carotene (30%), total ascorbic acid (58%), and total anthocyanins (20%); however, with a concomitant significant yield reduction of 47% [74]. On the other hand, moderate nutrient stress (half-strength nutrient solution-NS) applied to red Salanova butterhead lettuce (Lactuca sativa var. capitata) enhanced the concentrations of total ascorbic acid, total phenolic acids, and anthocyanins by 266%, 162%, and 380%, respectively, compared to the control, grown under full-strength NS [75]. For the above reasons, mild salinity, unbalanced mineral nutrition, or complete nutrient deprivation in the growth solution of soil-less culture systems for microscale vegetable production may prove helpful to naturally modulate the levels of functional compounds, such as ascorbate, carotenoids, and phenols. Moreover, it may also curtail anti-nutrients such as nitrate [74]. Sulfur is essential in the biosynthesis of secondary metabolites, such as glucosinolates in Brassica crops. Levels of sulfur and/or nitrogen nutrition during plant growth may result in significant changes in the phenolic content of edible plant parts, especially flavonoids and hydroxycinnamic acid derivatives [38]. Sulfur fertilization significantly improved the antioxidant activity of two ecotypes of spring broccoli, also known as Italian turnip (Brassica rapa subsp. sylvestris var. esculenta). It was associated with a genotype-dependent significant reduction in leaf nitrate content [76]. Environmental shocks such as high light (exposure to a light intensity of 700 µmol m−2 s−1 for 1 day) and chilling (exposure to 4 °C at a light intensity of 120 µmol m−2 s−1 for 1 day) enhanced the total phenolic content in sprouts of alfalfa (Medicago sativa), broccoli (Brassica oleracea var. italica), and radish (Raphanus sativus) [77]. The enhanced phenolic content was correlated with higher antioxidant activity, and dry biomass accumulation was unaffected. High light produced a more robust response than chilling in enhancing the content of individual phenolic compounds. Similarly, kale sprouts (B. oleracea var. acephala) exposed to low-temperature stress (growth temperature of 8 °C with intermittent freezing for one hour at −8 °C) increased the total content of phenolic acids and glucosinolates. However, such a treatment should be used with caution, as it also led to a significant decrease in the content of carotenoids and total flavonoids [78]. Radiation with short wavelengths, such as ultraviolet (UV) lights (200–400 nm), stimulates the production of pigments that absorb light and enhance leaf coloring, such as chlorophylls and carotenoids [79]. UV radiation may also induce physiological and metabolic stress responses in plants, such as the production of antioxidant systems, the activation of reparative enzymes, the expression of genes involved in UV protection and repair, and the accumulation of UV-absorbing compounds (e.g., phenolics and carotenoids) and defense-related (e.g., glucosinolates) phytochemicals [80]. This effect of UV light has been applied to broccoli sprouts to induce the biosynthesis and accumulation of flavonoids and glucosinolates [81]. Within 24 h after application of low UV-B (280–320 nm) doses, the flavonoids kaempferol and quercetin and glucosinolates accumulated in broccoli sprouts. A single exposure of broccoli sprouts to UV-B and UV-A (320–400 nm) for 120 min before the harvest was shown to enhance the phenolic and glucosinolate contents [82]. A synergistic effect in the accumulation of neoglucobrassicin was observed by exposing broccoli sprouts to a combination of UV irradiation and sprays of the phytohormone methyl jasmonate (25 µM). A single application of UV-B triggered the production of aliphatic or specific indole glucosinolates [82]. Exposing kale (Brassica oleracea var. sabellica) sprouts to periodical low UV-B treatments on days 3, 5, 7, and 10 of sprouting, with the four treatments reaching a total dose of either 10 or 15 kJ m−2, is a helpful tool to stimulate the biosynthesis of phytochemicals without compromising sprout growth [83]. During sprouting, repeated UV-B treatments increased the total phenolic content of kale sprouts by 30%, stimulating the synthesis of glucosinolates (glucoraphanin and glucobrassicin) by 30% and enhancing the antioxidant activity by 20%. Therefore, the periodic application of low UV-B doses during sprout growth can optimize the content of phytochemicals in microscale vegetables. Postharvest exposure of broccoli and radish sprouts to abiotic stress treatment in the form of UV-B radiation enhanced total phenolic content (TPC) and total antioxidant capacity (TAC) after a shelf life of 10 days at 4 °C [84]. UV-B treatment also enhanced the glucosinolates content of both crops by about 30%, while the content of sulforaphane increased by 38% in broccoli sprouts and 72% in radish sprouts. Zlotek et al. [85] compared the effect of thermal (2-day-old sprouts exposed for 2 h to 40 °C), osmotic (NaCl exposure), and oxidative (H2O2 exposure) stresses on adzuki bean (Vigna angularis) sprouts. Their research revealed that only thermal stress enhanced the antioxidant activity of extracts obtained from the adzuki bean seed coat [85]. Similarly, Świeca et al. [86] were able to demonstrate that both low (4 °C) and high (40 °C) temperature stress may cause an increase in the content of polyphenols and enhance the antioxidant properties of lentil (Lens culinaris) sprouts. The preharvest treatment of broccoli microgreens with 10 mM calcium chloride (CaCl2) to extend their shelf life led to a significant increase in aliphatic and indolic glucosinolates [87]. The raised glucosinolate (GLS) levels may have been responsible for the strengthened stress tolerance and defense mechanisms of broccoli microgreens, which resulted in delayed postharvest decay of the microgreens. This positive effect of a 10 mM calcium chloride (CaCl2) preharvest treatment was confirmed in experiments conducted by Lu et al. [88], which showed a significant increase in aliphatic glucosinolates levels and an overall improvement in visual quality and a longer storage life of broccoli microgreens. The above examples have shown that applying environmental stresses might be a viable approach to enhance the health-promoting qualities of microscale vegetables. However, the most promising type of environmental stress and its intensity require crop-specific research.5.3. Seed Priming and Biostimulants
Seed priming enhances seed germination, seedling growth, plant establishment, and crop performance. Priming techniques include hydro-priming (soaking seeds in water); osmo-priming (soaking seeds in osmotic solutions, such as polyethylene glycol); halo-priming (soaking seeds in sodium and potassium salts); solid matrix priming (mixing seeds with solid or semi-solid material and a specified amount of water); biopriming (coating seeds with beneficial fungi or bacteria); and treatment with plant growth regulators that are incorporated into the priming medium [89][90][91][92]. Seed priming with potassium nitrate (KNO3) improves seedling establishment and plant vigor [93]. Sprouts of three Medicago species treated with KNO3 showed increased total phenolic and flavonoid contents and enhanced antioxidant and antidiabetic activities [94]. The response of KNO3 priming was species-specific, with Medicago intertexta showing the highest antioxidant and antidiabetic activities, followed by M. polymorpha and M. indicus. The phytohormones jasmonic acid and methyl jasmonate (MeJA) (25–250 μM) and the amino acid DL-methionine (1–10 mM) were used as elicitors to enhance the total glucosinolate content of broccoli and radish sprouts [95]. The most effective treatments consisted of 24 h imbibition of seeds in priming solution, followed by exogenous sprays of elicitors on the cotyledons from days 4 to 7 of sprouting. MeJA priming in combination with exogenous sprays of elicitors led to the most significant increases of total glucosinolate content, from 34% to 100% in broccoli sprouts and from 45% to 118% in radish sprouts. Commercial biostimulants containing beneficial fungi or bacteria promoting plant growth are often recommended as a sustainable strategy to increase plant performance productivity and produce quality under environmental stresses aggravated by climate change [96]. Plant biostimulants are commonly defined as “Substance(s) and/or micro-organisms whose function is to stimulate natural processes that enhance nutrient uptake, nutrient use efficiency, tolerance to abiotic stress, and crop quality” [97]. Bioactive molecules in commercial biostimulants enhance the capability of plants to overcome adverse environmental conditions through their action on primary or secondary plant metabolism [98]. In addition, the presence of phytohormones and other secondary metabolites, vitamins, antioxidants, and inorganic nutrients in the extract of biostimulants may affect plant growth and production directly by enhancing plant tolerance against abiotic stresses [99]. The use of exogenous fungal polysaccharide elicitors obtained from the endophytic fungus Bionectra pityrodes (race Fat6) enhanced the sprout growth and flavonoid (rutin, quercetin) production of tartary buckwheat (Fagopyrum tataricum) [100]. Seed inoculation of common buckwheat (Fagopyrum esculentum) with the endophytic bacterium Herbaspirillum sp. (isolate ST-B2), isolated from common buckwheat seedling stems, enhanced the growth of sprouts and microgreens, promoted root elongation, and increased sprout and microgreen yields [101]. Soaking common buckwheat seeds in a solution containing Ecklonia maxima algae extract, which is known to enhance plant tolerance to abiotic stressors and plant growth, promoted the accumulation of dry matter in sprouts [35]. Buckwheat sprouts grown from seeds soaked in a solution containing nitrophenols, which occur naturally in plants (Biostimulant Asahi SL), and Pythium oligandrum oospores, showed a significantly higher level of crude protein [35]. P. oligandrum is a common oomycete found in soils worldwide and has a beneficial effect on pathogen control and induces resistance in the host plant [102]. Therefore, using fungal, bacterial, or other elicitors could be an efficient strategy for improving the nutritional and functional quality of sprouts and microgreens. During recent years, the study and use of plant biostimulants has been steadily growing. They may be applied singly or in combination, and there may be synergistic and additive effects of microbial and non-microbial plant biostimulators. Meanwhile, the design and development of the second generation of plant biostimulants are underway with specific modes of action to render agriculture more sustainable and resilient [97].6. Conclusions
Sprouts and microgreens are novel functional food sources with great potential for sustainably diversifying global food systems, promoting human health, and facilitating the access of a steadily growing urban population to fresh microscale vegetables. These novel food sources have vivid colors, exciting textures, and diverse flavors and tastes, and they can be purchased in supermarkets or even home-grown for daily harvesting as needed. Furthermore, due to their short growth cycle, these nutrient-dense food sources can be produced with minimal input, without using pesticides; hence, they have low environmental impacts and a broad acceptance among health-conscious consumers. Furthermore, as sprouts and microgreens are usually consumed raw, there is hardly a loss or degradation of heat-sensitive micronutrients or vitamins through food processing.References
- Micha, R.; Mannar, V.; Afshin, A.; Allemandi, L.; Baker, P.; Battersby, J.; Bhutta, Z.; Chen, K.; Corvalan, C.; Di Cesare, M.; et al. 2020 Global Nutrition Report: Action on Equity to End Malnutrition; Development Initiatives: Washington, DC, USA, 2020; Available online: http://eprints.mdx.ac.uk/30645/ (accessed on 12 November 2021).
- Neufeld, L.M.; Hendriks, S.; Hugas, M. Healthy Diet: A Definition for the United Nations Food Systems Summit 2021. A Paper from the Scientific Group of the UN Food Systems Summit. 2021. Available online: https://www.un.org/sites/un2.un.org/files/healthy_diet_scientific_group_march-2021.pdf (accessed on 15 November 2021).
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 18 November 2021).
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 2018, 392, 1072–1088.
- Vermeulen, S.J.; Park, T.; Khoury, C.K.; Béné, C. Changing diets and the transformation of the global food system. Ann. N. Y. Acad. Sci. 2020, 1478, 3–17.
- Padulosi, S.; Sthapit, B.; Lamers, H.; Kennedy, G.; Hunter, D. Horticultural biodiversity to attain sustainable food and nutrition security. Acta Hortic. 2018, 1205, 21–34.
- World Health Organization (WHO). Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. Available online: https://www.who.int/elena/titles/fruit_vegetables_ncds/en/ (accessed on 19 November 2021).
- Oyebode, O.; Gordon-Dseagu, V.; Walker, A.; Mindell, J.S. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: Analysis of Health Survey for England data. J. Epidemiol. Community Health 2014, 68, 856–862.
- Ebert, A.W. Sprouts, microgreens, and edible flowers: The potential for high value specialty produce in Asia. In Proceedings of the SEAVEG 2012 High Value Vegetables Southeast Asia Production, Supply Demand, Chiang Mai, Thailand, 16 September 2013; pp. 216–227.
- Voinea, L.; Vrânceanu, D.M.; Filip, A.; Popescu, D.V.; Negrea, T.M.; Dina, R. Research on food behavior in Romania from the perspective of supporting healthy eating habits. Sustainability 2019, 11, 5255.
- Ebert, A.W. High value specialty vegetable produce. In Handbook of Vegetables, 1st ed.; Peter, K.V., Hazra, P., Eds.; Studium Press LLC.: Houston, TX, USA, 2015; Chapter 4; Volume 2, pp. 119–143.
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115.
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, microgreens and “baby leaf” vegetables. In BT-Minimally Processed Refrigerated Fruits and Vegetables; Food Engineering Series; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7018-6.
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421.
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118.
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424.
- Vidal-Valverde, C.; Frias, J.; Sierra, I.; Blazquez, I.; Lambein, F.; Kuo, Y.H. New functional legume foods by germination: Effect on the nutritive value of beans, lentils and peas. Eur. Food Res. Technol. 2002, 215, 472–477.
- Márton, M.; Mandoki, Z.S.; Csapo-Kiss, Z.S.; Csapo, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae 2010, 3, 81–117.
- Wanasundara, P.; Shahidi, F.; Brosnan, M.E. Changes in flax (Linum usitatissimum) seed nitrogenous compounds during germination. Food Chem. 1999, 65, 289–295.
- Lee, J.S.; Pill, W.G.; Cobb, B.B.; Olszewski, M. Seed treatments to advance greenhouse establishment of beet and chard microgreens. J. Hort. Sci. Biotechnol. 2004, 79, 565–570.
- Di Gioia, F.; Mininni, C.; Santamaria, P. How to grow microgreens. In Microgreens: Microgreens: Novel Fresh and Functional Food to Explore All the Value of Biodiversity; Di Gioia, F., Santamaria, P., Eds.; ECO-Logica: Bari, Italy, 2015; pp. 51–79.
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and mineral composition of three wild leafy species: A comparison between microgreens and baby greens. Foods 2019, 8, 487.
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop; HS1164, rev. 9/2020, IFAS Extension; University of Florida: Gainesville, FL, USA, 2020.
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882.
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66.
- Verlinden, S. Microgreens: Definitions, Product Types, and Production Practices. Hortic. Rev. 2020, 47, 85–124.
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148.
- Ghani, M.; Kulkarni, K.P.; Song, J.T.; Shannon, J.G.; Lee, J.D. Soybean sprouts: A review of nutrient composition, health benefits and genetic variation. Plant Breed. Biotechnol. 2016, 4, 398–412.
- Butkutė, B.; Taujenis, L.; Norkevičienė, E. Small-seeded legumes as a novel food source. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds-sprouted seeds-microgreens. Molecules 2019, 24, 133.
- Kurian, M.S.; Megha, P.R. Assessment of variation in nutrient concentration and antioxidant activity of raw seeds, sprouts and microgreens of Vigna radiata (L.) Wilczek and Cicer arietinum L. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2020; Volume 2263, 030005p.
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.A.; Van den Broeck, H.C.; Brouns, F.J.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328.
- Pirzadah, T.B.; Malik, B. Pseudocereals as super foods of 21st century: Recent technological interventions. J. Agric. Food Res. 2020, 2, 100052.
- Ebert, A.W.; Wu, T.H.; Yang, R.Y. Amaranth sprouts and microgreens—A homestead vegetable production option to enhance food and nutrition security in the rural-urban continuum. In Proceedings of the Regional Symposium on Sustaining Small-Scale Vegetable Production and Marketing Systems for Food and Nutrition Security (SEAVEG 2014), Bangkok, Thailand, 25–27 February 2014; pp. 25–27.
- Janovská, D.; Stocková, L.; Stehno, Z. Evaluation of buckwheat sprouts as microgreens. Acta Agric. Slov. 2010, 95, 157.
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wu, X.; Tan, M.; Nie, Z.; Wu, Q.; et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752.
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and microorganisms boost the nutritional composition of buckwheat (Fagopyrum esculentum Moench) sprouts. Agronomy 2019, 9, 469.
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478.
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43.
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998.
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.; Chen, P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2021, 2, 101–113.
- Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family. Foods 2021, 10, 427.
- Ng, T.B.; Ng, C.C.W.; Wong, J.H. Health benefits of Brassica species. In Brassicaceae: Characterization, Functional Genomics and Health Benefits; Lang, M., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 1–18.
- Sanlier, N.; Guler, S.M. The benefits of Brassica vegetables on human health. J. Hum. Health Res. 2018, 1, 1–13.
- Francisco, M.; Velasco, P.; Moreno, D.A.; García-Viguera, C.; Cartea, M.E. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res. Int. 2010, 43, 1455–1463.
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159.
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; de Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Mol. Nutr. Food Res. 2009, 53, S219–S265.
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1.
- Rose, P.; Huang, Q.; Ong, C.N.; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2005, 209, 105–113.
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372.
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous Vegetables and Human Cancer Risk: Epidemiologic Evidence and Mechanistic Basis. Pharmacol. Res. 2007, 55, 224.
- De la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250.
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer sensory perception and acceptance of an emerging functional food crop. J. Food Sci. 2020, 85, 926–935.
- Ghoora, M.D.; Srividya, N. Micro-farming of greens: A viable enterprise for enhancing economic, food and nutritional security of farmers. Int. J. Nutr. Agric. Res. 2018, 5, 10–16.
- Dimita, R.; Min Allah, S.; Luvisi, A.; Greco, D.; De Bellis, L.; Accogli, R.; Mininni, C.; Negro, C. Volatile Compounds and Total Phenolic Content of Perilla frutescens at Microgreens and Mature Stages. Horticulturae 2022, 8, 71.
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495.
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043.
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651.
- Trifunovic, S.; Topalovic, A.; Knezevic, M.; Vajs, V. Free radicals and antioxidants: Antioxidative and other properties of Swiss chard (Beta vulgaris L. subsp. Cicla). Poljopr. Sumar. 2015, 61, 73.
- Davis, D.R.; Epp, M.D.; Riordan, H.D. Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J. Am. Coll. Nutr. 2004, 23, 669–682.
- Garvin, D.F.; Welch, R.M.; Finely, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220.
- Fan, M.-S.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324.
- White, P.J.; Bradshaw, J.E.; Finlay, M.; Dale, B.; Ramsay, G.; Hammond, J.P.; Broadley, M.R. Relationships between yield and mineral concentrations in potato tubers. Hort Sci. 2009, 44, 6–11.
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103.
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in Brassica oleracea L. novel foods: Sprouts, microgreens and baby leaves. Agronomy 2020, 10, 782.
- Chatzopoulou, E.; Carocho, M.; Di Gioia, F.; Petropoulos, S.A. The beneficial health effects of vegetables and wild edible greens: The case of the Mediterranean diet and its sustainability. Appl. Sci. 2020, 10, 9144.
- Romojaro, A.; Botella, M.Á.; Obón, C.; Pretel, M.T. Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int. J. Food Sci. Nutr. 2013, 64, 944–952.
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J. Agric. Food Chem. 2008, 56, 1912–1920.
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76.
- Hong, J.; Gruda, N.S. The potential of introduction of Asian vegetables in Europe. Horticulturae 2020, 6, 38.
- Song, J.S.; Jung, S.; Jee, S.; Yoon, J.W.; Byeon, Y.S.; Park, S.; Kim, S.B. Growth and bioactive phytochemicals of Panax ginseng sprouts grown in an aeroponic system using plasma-treated water as the nitrogen source. Sci. Rep. 2021, 11, 2924.
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530.
- Weber, C.F. Nutrient concentration of cabbage and lettuce microgreens grown on vermicompost and hydroponic growing pads. J. Hortic. 2016, 3, 190.
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42.
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191.
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables Through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1254.
- Benincasa, P.; D’Amato, R.; Falcinelli, B.; Troni, E.; Fontanella, M.C.; Frusciante, S.; Guiducci, M.; Beone, G.M.; Businelli, D.; Diretto, G. Grain Endogenous Selenium and Moderate Salt Stress Work as Synergic Elicitors in the Enrichment of Bioactive Compounds in Maize Sprouts. Agronomy 2020, 10, 735.
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Gaspari, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Nutrient Supplementation Configures the Bioactive Profile and Production Characteristics of Three Brassica L. Microgreens Species Grown in Peat-Based Media. Agronomy 2021, 11, 346.
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972.
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp. sylvestris. Eur. J. Agron. 2007, 26, 418–424.
- Oh, M.M.; Rajashekar, C.B. Antioxidant content of edible sprouts: Effects of environmental shocks. J. Sci. Food Agric. 2009, 89, 2221–2227.
- Šamec, D.; Ljubej, V.; Redovnikovi’c, I.R.; Fistani’c, S.; Salopek-Sondi, B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts . Foods 2022, 11, 264.
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and Visible Spectrum LED Lighting as Abiotic Elicitors of Bioactive Compounds in Sprouts, Microgreens, and Baby Leaves—A Comprehensive Review including Their Mode of Action. Foods 2022, 11, 265.
- Jenkins, G.I.; Brown, B.A. UV-B perception and signal transduction. In Light and Plant Development; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 155–182.
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560.
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330.
- Castillejo, N.; Martínez-Zamora, L.; Artés–Hernández, F. Periodical UV-B Radiation Hormesis in Biosynthesis of Kale Sprouts Nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285.
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés-Hernández, F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304.
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic Assessment Reveals an Elevated Level of Glucosinolate Content in CaCl2 Treated Broccoli Microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868.
- Lu, Y.; Dong, W.; Alcazar, J.; Yang, T.; Luo, Y.; Wang, Q.; Chen, P. Effect of preharvest CaCl2 spray and postharvest UV-B radiation on storage quality of broccoli microgreens, a richer source of glucosinolates. J. Food Compos. Anal. 2018, 67, 55–62.
- Mercado, M.F.O.; Fernandez, P.G. Solid matrix priming of soybean seeds. Philipp. J. Crop Sci. 2002, 27, 27–35.
- Pandita, V.K.; Anand, A.; Nagarajan, S.; Seth, R.; Sinha, S.N. Solid matrix priming improves seed emergence and crop performance in okra. Seed Sci. Technol. 2010, 38, 665–674.
- Mondal, S.; Bose, B. An impact of seed priming on disease resistance: A review. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R., Upadhyay, R., Dubey, N., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 193–203.
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of seed priming with potassium nitrate on the performance of tomato. Agriculture 2020, 10, 498.
- Zrig, A.; Saleh, A.; Hamouda, F.; Okla, M.K.; Al-Qahtani, W.H.; Alwasel, Y.A.; Al-Hashimi, A.; Hegab, M.Y.; Hassan, A.H.; AbdElgawad, H. Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species. Plants 2022, 11, 71.
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319.
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794.
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655.
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306.
- Ali, Q.; Shehzad, F.; Waseem, M.; Shahid, S.; Hussain, A.I.; Haider, M.Z.; Habib, N.; Hussain, S.M.; Javed, M.T.; Perveen, R. Plant-based biostimulants and plant stress responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 625–661.
- Zhao, J.L.; Zou, L.; Zhong, L.Y.; Peng, L.X.; Ying, P.L.; Tan, M.L.; Zhao, G. Effects of polysaccharide elicitors from endophytic Bionectria pityrodes Fat6 on the growth and flavonoid production in tartary buckwheat sprout cultures. Cereal Res. Commun. 2015, 43, 661–671.
- Briatia, X.; Jomduang, S.; Park, C.H.; Lumyong, S.; Kanpiengjai, A.; Khanongnuch, C. Enhancing growth of buckwheat sprouts and microgreens by endophytic bacterium inoculation. Int. J. Agric. Biol. 2017, 19, 374–380.
- Gerbore, J.; Vallance, J.; Yacoub, A.; Delmotte, F.; Grizard, D.; Regnault-Roger, C.; Rey, P. Characterization of Pythium oligandrum populations that colonize the rhizosphere of vines from the Bordeaux region. FEMS Microbiol. Ecol. 2014, 90, 153–167.
