Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Umer Waqas.
Earthen materials such as rocks have a wide range of applications in various rock-engineering related domains, including rock slope stabilization, rock drilling, tunneling and excavation, coal gasification, nuclear waste repositories, geothermal energy extraction, construction material, and foundation engineering. Rocks are heterogeneous, anisotropic, and aggregate of different minerals.
- mineral dilatancy
- thermal cracking
1. Introduction
Earthen materials such as rocks have a wide range of applications in various rock-engineering related domains, including rock slope stabilization, rock drilling, tunneling and excavation, coal gasification, nuclear waste repositories, geothermal energy extraction, construction material, and foundation engineering [1,2,3,4][1][2][3][4]. Rocks are heterogeneous, anisotropic, and aggregate of different minerals. They are not perfectly elastic material but rather brittle in nature. However, deep-seated rocks show ductility under high pressure and temperature conditions [5]. The rock mass exposed to the surface is found discontinuous due to its weathering. Temperature is one of the most important weathering agents that significantly alter the engineering properties of the rock mass. Construction of sensitive structures, such as skyscrapers, dams, nuclear power plants, tunnels, etc., in the thermally deteriorated rock mass is taken into account as a major challenge. These structures may experience severe damage or reduction in their service life under the adverse effects of altered engineering properties of the thermally damaged rock mass [6]. Therefore, the study of thermal effects on rock properties has been garnering attention for the last few decades.
Carbonate rocks are a sub-class of sedimentary rocks and abundantly found on the upper Earth’s crust. Limestone and dolostone are the two major types of carbonate rocks and have a wide range of applications in the construction, cement, glass, mining, and petroleum industries [7]. Several researchers have investigated the engineering behavior of carbonate rocks subjected to various temperature ranges between ambient temperature and high temperature (20–1000 °C) [6,7,8,9,10,11,12,13][6][7][8][9][10][11][12][13]. They have noticed that limestone and dolostone under high temperatures experience mineralogical alteration, inter-granular and intra-granular cracking, reduction in dynamic–mechanical strength parameters, and appreciation in porosity and permeability.
In high-temperature rock mechanics and geotechnics, the evaluation of the engineering characteristics of carbonate rocks is of great interest. For example, dimension stones obtained from carbonate or silicate rocks are important construction materials. In a building fire event, their temperature may rise above 800 °C [14]. The underground coal gasification process may increase the temperature of host carbonate rocks up to 1500 °C [15]. This process not only significantly damages host rocks but also releases oxides of nitrogen, carbon, sulfur, etc., in the environment. Similarly, during a plate tectonic event (at depth > 40 km and 500–850 °C), thick deposits of carbonate rocks at the subduction zone liberate excessive carbon dioxide, which is one of the major global warming factors [16]. Furthermore, magmatic activities, such as contact metamorphism, increase the temperature of country rocks from 300 to 800 °C [17]. At a shallow depth, the decay of radioactive elements in their repositories heats the host rocks from 50 to 250 °C [7]. The level of temperature and pressure increases with depth. Therefore, the extraction of geothermal energy and hydrocarbons from deep-seated carbonate rocks requires special attention because of their altered geomechanical characteristics [18].
2. Thermal Dilatancy and Alteration in Rock Fabric
Carbonate rocks obtained from various sources can differ significantly in their texture, depositional environment, chemical composition, crystal structure, and mineral geometry [19]. Limestones contain more than 50% calcite and a trace amount of a variety of minerals, including quartz, feldspar, pyrite, siderite, micrite, clay minerals, and other materials. On the other hand, dolostone is composed of the dolomitization process in which calcite transforms into magnesium-rich calcium carbonate. It contains dolomite as a primary mineral and a trivial amount of quartz, mica, iron oxide, and clay minerals [20]. They are chemically reactive substances and show large variations in their chemical reactions. Microstructure patterns and impurities, such as silica, iron, magnesium, manganese, sodium, potassium, etc., considerably affect their chemical reactivity [19]. The calcite and dolomite minerals belong to the hexagonal-rhombohedral crystal system. In this crystal system, the hexagonal unit cell is placed over the rhombohedral unit cell [21]. Calcite mineral has ordered planes of Ca2+ attached with the CO32− groups orthogonal to the c-axis. Whereas, dolomite mineral exhibits a well-defined order of alternating planes of Ca2+ and Mg2+ bonded with the CO32− groups perpendicular to the c-axis [22].2.1. Thermal Decomposition of Calcite and Dolomite
The investigation of thermal decomposition of the primary carbonate minerals is of great interest. It develops a solid background rationale to anticipate the possible reason behind microstructural variations in carbonate rocks under a thermal environment. The reaction kinetics explains the decomposition of carbonate minerals into their respective constituents at elevated temperatures.
The rate of reaction is controlled by some important factors, such as heat transfer rate, mass transfer rate, or their combination [23]. It is evident from past studies that particle size, crystal structure, the order of atoms in a unit cell, possible impurities, and crystal habit substantially affect the thermal transport mechanism and mineral dilatancy [23,24][23][24]. Considerable variations are found in the kinetic parameters of thermally decomposed carbonate minerals. For example, a great discrepancy is reported in the literature regarding the activation energy values of calcite (155–222 kJ mol−1) and dolomite (146–440 kJ mol−1) [25]. As the temperature increases, the calcite starts to deform due to thermal expansion and chemical reactions. It decomposes into calcium oxide (cubic crystal system) with the liberation of carbon dioxide at a temperature greater than 600 °C [26]. Chemically, it can be expressed as follows:
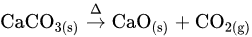 (1)
(1)
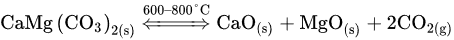 (2)
(2)
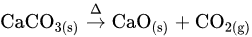 (1)
(1)The hexagonal–rhombohedral crystal system of dolomite (i.e., CaMg(CO3)2) begins to deform because of the dislocation of cations and anions. Its thermal decomposition produces calcium oxide (cubic crystal system), magnesium oxide (cubic crystal system), and carbon dioxide gas [25]. Several models have been developed to understand the thermal decomposition mechanism for both natural and synthetic dolomites [27,28,29,30,31,32][27][28][29][30][31][32]. The single-step reaction for the dolomite decomposition is described below:
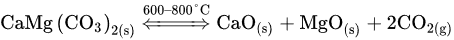 (2)
(2)Another proposed model suggests that the thermal decomposition of a dolomite mineral completes in more than one step [33]. In the first step, it breaks into magnesium carbonate and calcium carbonate. In the second step, unstable magnesium carbonate decomposes into magnesium oxide. Finally, calcium carbonate turns into its respective metallic oxide against increasing temperature.
To study thermal damage characteristics and decomposition of carbonate minerals, several techniques have been utilized, such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), differential thermal analysis (DTA), thermo-balance, scanning electron microscopy, optical microscopy, X-ray diffraction, high-temperature X-ray diffraction, etc. [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48]. A summary of major developments and employed quantitative methods regarding the decomposition of carbonate minerals is provided in Table 1.
Table 1.
Summary of major developments regarding the thermal decomposition of primary carbonate minerals.
| Mineral Type | Major Developments | Reference |
|---|---|---|
| Calcite | The rate of mass loss was studied using isothermal and dynamic methods. | [34] |
| Calcite | A comparative study was conducted using isothermal–dynamic techniques and thermogravimetric analysis. | [35] |
| Calcite | Morphological variations were studied in polycrystalline CaCO3 under temperature and pressure. | [36] |
| Dolomite | Thermal decomposition and weight loss analysis was carried out under non-isothermal conditions using in situ X-ray diffraction and thermogravimetry. | [37] |
| Calcite | Reaction rate constants were determined based on the grain model using thin slab-type pellets. | [38] |
| Calcite | Thermal decomposition was analyzed using dynamic X-ray diffraction under the effect of steam and CO2. | [39] |
| Dolomite | Prediction of rate of reaction using stoichiometric analysis and thermogravimetric analysis. | [27] |
| Calcite | Thermal decomposition was investigated using thermogravimetric analysis subjected to non-isothermal conditions. | [40] |
| Calcite | The kinetic parameters were obtained from a new method that avoids the Arrhenius equation. | [41] |
| Dolomite | Thermo-mechanical damage was examined under intensive grinding using X-ray diffraction and thermal analysis. | [42] |
| Calcite | The solid-state transformation was evaluated using thermogravimetric analysis, evolved gas analysis-mass spectrometry, and high-temperature XRD. | [43] |
| Dolomite | The thermal decomposition mechanism was explained in detail using thermogravimetry and X-ray powder diffraction. | [33] |
| Dolomite | Thermal expansion and decomposition behavior were investigated using thermogravimetric analysis, differential thermal analysis, XRD, and scanning electron microscopy. | [29] |
]. Each rock-forming mineral has a specific value of the coefficient of thermal expansion. Mineral elongation under thermal stresses increases the particle contact surface area and causes microstructural changes in the rock matrix that adversely affect the mechanical, physical, and dynamic properties of rocks [60]. The phenomenon of thermal damage to rocks can be ascertained by observing the microscopic changes in rocks, such as mineral expansion under thermal stresses, grain boundary conditions, the density of intergranular and intragranular cracks, amount of the induced thermal strain, and mineral resistance to destruction [61].
Temperature levels and time of exposure are both factors that play an important role in the thermal degradation of different rocks. For example, in the case of crystalline rocks at a low temperature, no significant changes are observed in their internal structure because of their resistant mineral composition. At a moderate level of temperature (300–500 °C), minerals start to expand and make closures at grain boundaries that lead to a reduction in the void spaces. At a high level of temperature (>500 °C), thermal stresses in the rocks exceed the threshold limit of minerals’ coefficient of thermal expansion, which causes mineral damage and relaxation at their contact boundaries. Researchers observed that mineral expansion in crystalline rocks occurs at a temperature ranging from 400 °C to 600 °C, and, beyond this temperature, rocks start to deform plastically [62,63][62][63]. On the other hand, in the case of carbonate rocks, at a low level of heating (<300 °C), the relaxation phenomenon starts at the grain contact boundaries because their minerals and bonding agents are less resistant to thermal damage. On heating limestones at temperatures > 600 °C, the emission of carbon dioxide with the decomposition of calcium carbonates weakens the limestone [57].
Advanced techniques such as X-ray diffraction (XRD), X-ray fluorescence (XRF), computerized tomography scanning (CT), and scanning electron microscopy (SEM) have been used widely in high-temperature rock mechanics to investigate microscopic variations in rocks that give an idea to understand macroscopic changes [64,65,66,67][64][65][66][67]. Furthermore, a summary of the major findings regarding the microscopic variations in thermally damaged carbonate rocks is described in Table 2.
Table 2.
Summary of major findings regarding the microscopic evaluation of the thermally damaged carbonate rocks.
| Temperature Range | Major Findings | Reference |
|---|---|---|
| 200–800 °C | They analyzed the thermally treated limestones using scanning electron microscopy. They showed orientation of thermal tension and shear cracks developed in limestone. The cracks were straight, curved, parallel, vertical, oblique, and crossed layers. | [64] |
| 100–500 °C | They studied the monomineralic carbonate rocks subjected to various temperature ranges. They observed that, in these kinds of carbonate rocks, thermal damage was the function of anisotropic dilation of calcite and shrinkage of clay minerals. The mineral expansion was observed at a temperature range of 100–200 °C, whereas intergranular and intragranular cracking was noted at 300–500 °C. | [13] |
| 25–600 °C | They demonstrated the thermal deterioration of the limestone in terms of spectral reflectance. They found that, at an initial level of temperature, mineral expansion under elastic constraints increased the spectral reflectance, and, at a temperature above 500 °C, the thermal degradation of minerals decreased their spectral reflectance. | [20] |
| 20–1000 °C | He investigated the effect of mineral crystal structures on the thermal behavior of carbonate rocks. In the case of dolostone, he observed that larger crystals of dolomite minerals decomposed more than the dolostone containing the smaller size dolomite crystals. | [65] |
| 25–800 °C. | XRF technique was used to investigate the microstructural changes in limestones. They noticed an appreciable alteration in the percentage of the mineral content at a temperature window of 400–700 °C. | |
| Dolomite | ||
| Stoichometric ordered and disordered single crytal dolomite was studied using X-ray diffraction under high pressure and temperature conditions. | ||
| [ | ||
| 44 | ||
| ] | ||
| Dolomite | Investigation of kinetics of isothermal and non-isothermal decompositions. | [25] |
| Calcite | A simulated model was presented that effectively predicted the conversion time curve and described the calcination–carbonation cycle after performing thermogravimetric analysis. | [45] |
| Dolomite | Differential scanning calorimetry and thermogravimetric analysis based on non-isothermal calcination carried out under varying CO2–air environments. | [46] |
| Calcite | Thermo-physical decomposition was studied under equilibrium dynamic simulation. | [47] |
| Calcite | Parameters including unit cell volume alteration, thermal expansion, variations along lattice axis, and thermal strains were studied using high-temperature X-ray powder diffraction. | [26] |
| Calcite | Thermal decomposition analysis was performed to validate improved reaction kinetic equation based on the pore structure model. | [48] |
2.2. Reasons behind the Thermal Expansion
The crystal structure of a dolomite mineral is intermediate between calcite (CaCO3) and magnesite (MgCO3). However, it differs from calcite in terms of two aspects: alternating layers of calcium and magnesium in the unit cell and slight tilting of the carbonate group [49]. Thermal expansion of calcite along the a-axis is observed negative. On the other hand, in dolomite, it is measured positively along both the a-axis and c-axis [50]. Single carbonate crystals, such as calcite, show typical strength–temperature behavior (strength decrease with the temperature). Whereas, double carbonate crystals, such as dolomite, exhibit a different behavior that is shown by the calcite. It is attributed to the thermal vibration of the carbonate group that hinders the dislocation movement against increasing temperature [51].
In dolomite, the octahedral system of CaO6 and MgO6 plays an important role in stabilizing the crystal structure. The Ca-O bond length is found larger in dolomite as compared to that in calcite [49]. The variations in the bond length of Ca-O relative to temperature is different in both single carbonate crystals and double carbonate crystals. At the temperature range of 24 to 600 °C, in dolomite, a linear elongation in Ca-O bond length is observed at a faster rate. Whereas, in the case of calcite, an exponential trend is noted in the expansion of Ca-O bond length at a slower rate [52]. The bond strength can be expressed in terms of thermal expansion. Longer bonds (in dolomite) show less resistance and expand rapidly. There are different trends reported in the literature that explain the thermal behavior of polyhedral crystals in terms of volume expansion (VE) and quadratic elongation (QE). In dolomite, Ca-octahedron shows a very slight or no change in QE. Whereas, in Mg-octahedron, QE increases substantially. This behavior shows a contrast with the QE trends observed in calcite and magnesite. The QE under increasing temperature varies sharply for Ca-octahedron but, in the case of Mg-octahedron, exhibits no distortion [52,53][52][53]. The possible reason for such trends in these carbonate minerals is the thermal expansion between the oxygen atoms of octahedra at the basal edge and lateral edge. Furthermore, the Ca-octahedron of calcite shows less anisotropic thermal expansion. However, the thermal expansion in Mg-octahedron of dolomite displays more anisotropic character relative to magnesite [54]. Figure 1 illustrates the thermal effect on the interatomic distances of Ca-O and Mg-O in calcite, dolomite, and magnesite.
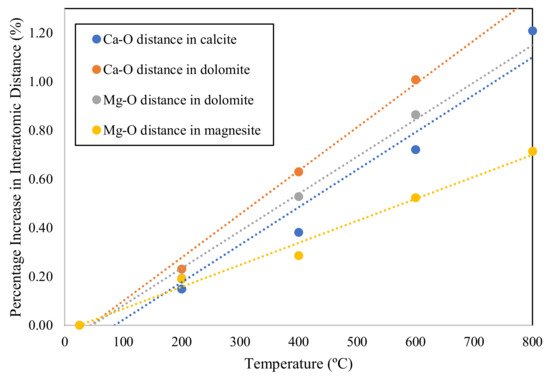

Figure 1. Percentage increase of thermal elongation in the bond length of Ca-O and Mg-O adapted from Reeder and Markgraf [52].
As concerns the thermal dislocation of the carbonate group in dolomite, it is intermediate between the calcite and magnesite. In calcite, the displacement of the carbonate group is the result of rotational disorder, which is the rotary oscillation about the three-fold axis [55]. In the case of dolomite and magnesite, the influence of the Mg-O bond confines the motion of the oxygen atoms. Therefore, the dislocation of the carbonate group in both dolomite and magnesite is less relative to that in calcite [56]. However, in magnesite, it is further less just because of the slight tilting of the basal plane. The displacement of the carbonate group contributes a minimum in the thermal deterioration of carbonate rocks. Previous studies show that, in calcite, the change in orientation of the carbonate group even at elevated temperature (i.e., 600 °C) is <0.5° as compared to its ambient conditions [49]. In dolomite, the adjacent layers share corners with different octahedra. In other words, the carbonate group makes the bond with unlike cations that further restrict its motion. This signifies that rotation of the carbonate group is not a decisive factor in the thermal deterioration of carbonate rocks. The thermal expansion of calcium and magnesium octahedral systems considerably affects the thermal damage of the carbonate rocks.
2.3. Thermal Cracking and Microstructural Variations
Carbonate rocks exposed to a temperature window of 500 °C to 1500 °C considerably experience an alteration in their mineral composition, mineral strength, physical structures, textural characteristics, and grain cementations [57]. Under the thermal environment, chemical processes, such as hydration or dehydration, red-ox reactions, deionization, mineral phase transformation, dissolution, and disappearance of bonding agents, alter the behavior of rocks to a great extent [58]. Rock–water interaction in geothermal systems, especially along the fault zones, recrystallizes the minerals through geochemical processes [59
| [ | ||
| 66 | ||
| ] | ||
| 20–800 °C | They studied microstructural variations in carbonate rocks and found no noticeable changes in the chemical composition at a temperature below 400 °C. Furthermore, they observed that calcite and dolostone were decomposed at 400–500 °C and clay minerals started to decay at a temperature above 500 °C. In the case of trace minerals and impurities, their concentration was decreased gradually up to 400 °C and then increased sharply above 600 °C. | [67] |
References
- Behnia, D.; Ahangari, K.; Moeinossadat, S.R. Modeling of shear wave velocity in limestone by soft computing methods. Int. J. Min. Sci. Technol. 2017, 27, 423–430.
- Lai, H.P.; Wang, S.Y.; Xie, Y.L. Experimental research on temperature field and structure performance under different lining water contents in a road tunnel fire. Tunn. Undergr. Space Technol. 2014, 43, 327–335.
- Nasseri, M.H.B.; Schubnel, A.; Young, R.P. Coupled evolutions of fracture toughness and elastic wave velocities at high crack density in thermally treated Westerly granite. Int. J. Rock Mech. Min. Sci. 2007, 44, 601–616.
- Heuze, F.E. High-temperature mechanical, physical, and thermal properties of granitic rocks—A review. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1983, 20, 3–10.
- Smith, B.M.; Reynolds, S.J.; Day, H.W.; Bodnar, R.J. Deep-seated fluid involvement in ductile-brittle deformation and mineralization, South Mountains metamorphic core complex, Arizona. Geol. Soc. Am. Bull. 1991, 103, 559–569.
- Waqas, U.; Ahmed, M.F. Prediction Modeling for the Estimation of Dynamic Elastic Young’s Modulus of Thermally Treated Sedimentary Rocks Using Linear–Nonlinear Regression Analysis, Regularization, and ANFIS. Rock Mech. Rock Eng. 2020, 53, 5411–5428.
- Idris, M.A. Effects of elevated temperature on physical and mechanical properties of carbonate rocks in South-Southern Nigeria. Min. Miner. Depos. 2018, 12, 20–27.
- Waqas, U.; Ahmed, M.F.; Arshad, M. Classification of the intact carbonate and silicate rocks based on their degree of thermal cracking using discriminant analysis. Bull. Eng. Geol. Environ. 2020, 79, 2607–2619.
- Yang, J.; Fu, L.Y.; Zhang, W.; Wang, Z. Mechanical property and thermal damage factor of limestone at high temperature. Int. J. Rock Mech. Min. Sci. 2019, 117, 11–19.
- Merriman, J.D.; Hofmeister, A.M.; Roy, D.J.; Whittington, A.G. Temperature-dependent thermal transport properties of carbonate minerals and rocks. Geosphere 2018, 14, 1961–1987.
- Nicolas, A.; Fortin, J.; Regnet, J.B.; Dimanov, A.; Guéguen, Y. Brittle and semi-brittle behaviors of a carbonate rock: Influence of water and temperature. Geophys. J. Int. 2016, 206, 438–456.
- Sengun, N. Influence of Thermal Damage on the Physical and Mechanical Properties of Carbonate Rocks. Arab. J. Geosci. 2014, 7, 5543–5551.
- Yavuz, H.; Demirdag, S.; Caran, S. Thermal effect on the physical properties of carbonate rocks. Int. J. Rock Mech. Min. Sci. 2010, 47, 94–103.
- Hajpál, M. Fire Damaged Stone Structures in Historical Monuments. Laboratory Analyses of Changes in Natural Stones by Heat Effect; CIB Publication: Salford, UK, 2010; pp. 164–173.
- Sury, M.; White, M.; Kirton, J.; Carr, P.; Woodbridge, R.; Mostade, M.; Chappell, R.; Hartwell, D.; Hunt, D.; Rendell, N. Review of Environmental Issues of Underground Coal Gasification–Best Practice Guide; Report No. COAl R273 DTi/Pub URN; U.S. Department of Energy: Oak Ridge, VA, USA, 2004; Volume 4, p. 1881.
- Behn, M.D.; Kelemen, P.B.; Hirth, G.; Hacker, B.R.; Massonne, H.J. Diapirs as the source of the sediment signature in arc lavas. Nat. Geosci. 2011, 4, 641–646.
- Philpotts, A.R.; Ague, J.J. Principles of Igneous and Metamorphic Petrology; Cambridge University Press: Cambridge, UK, 2009.
- Deegan, F.M.; Troll, V.R.; Freda, C.; Misiti, V.; Chadwick, J.P.; McLeod, C.L.; Davidson, J.P. Magma–carbonate interaction processes and associated CO2 release at Merapi Volcano, Indonesia: Insights from experimental petrology. J. Petrol. 2010, 51, 1027–1051.
- Kılıc, Ö. The influence of high temperatures on limestone P-wave velocity and Schmidt hammer strength. Int. J. Rock Mech. Min. Sci. 2006, 43, 980–986.
- González-Gómez, W.S.; Quintana, P.; May-Pat, A.; Avilés, F.; May-Crespo, J.; Alvarado-Gil, J.J. Thermal effects on the physical properties of limestones from the Yucatan Peninsula. Int. J. Rock Mech. Min. Sci. 2015, 75, 182–189.
- Lippmann, F. Sedimentary Carbonate Minerals; Springer Science & Business Media: Berlin, Germany, 1973.
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769.
- Asaki, Z.; Fukunaka, Y.; Nagase, T.; Kondo, Y. Thermal decomposition of limestone in a fluidized bed. Metall. Trans. 1974, 5, 381–390.
- Sanders, J.P.; Gallagher, P.K. Kinetic analyses using simultaneous TG/DSC measurements: Part II: Decomposition of calcium carbonate having different particle sizes. J. Therm. Anal. Calorim. 2005, 82, 659–664.
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248.
- Karunadasa, K.S.; Manoratne, C.H.; Pitawala, H.M.; Rajapakse, R.M. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28.
- Hartman, M.; Trnka, O.; Vesely, V.; Svoboda, K. Predicting the rate of thermal decomposition of dolomite. Chem. Eng. Sci. 1996, 51, 5229–5232.
- Barcina, L.M.; Espina, A.; Suárez, M.; Garcia, J.R.; Rodriguez, J. Characterization of monumental carbonate stones by thermal analysis (TG, DTG, and DSC). Ther. Acta 1997, 290, 181–189.
- Gunasekaran, S.; Anbalagan, G. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344.
- Kök, M.U.; Smykatz-Kloss, W. Characterization, correlation, and kinetics of dolomite samples as outlined by thermal methods. J. Therm. Anal. Calorim. 2008, 91, 565–568.
- Hossain, F.M.; Długogorski, B.Z.; Kennedy, E.M.; Belova, I.V.; Murch, G.E. First-principles study of the electronic, optical, and bonding properties in dolomite. Comput Mater Sci. 2011, 50, 1037–1042.
- Jiang, J.; Ye, J.; Zhang, G.; Gong, X.; Nie, L.; Liu, J. Polymorph and morphology control of CaCO3 via temperature and PEG during the decomposition of Ca(HCO3)2. J. Am. Ceram. Soc. 2012, 95, 3735–3738.
- Samtani, M.; Dollimore, D.; Wiburn, F.W.; Alexander, K. Isolation and identification of the intermediate and final products in the thermal decomposition of dolomite in an atmosphere of carbon dioxide. Ther. Acta 2001, 367, 285–295.
- Gallagher, P.; Johnson, D.W., Jr. The effects of sample size and heating rate on the kinetics of the thermal decomposition of CaCO3. Thermochim. Acta 1973, 6, 67–83.
- Caldwell, K.M.; Gallagher, P.K.; Johnson, D.W., Jr. Effect of thermal transport mechanisms on the thermal decomposition of CaCO3. Thermochim. Acta 1977, 18, 15–19.
- Maciejewski, M.; Oswald, H.R. Morphological observations on the thermal decomposition of calcium carbonate. Thermochim. Acta 1985, 85, 39–42.
- Engler, P.; Santana, M.W.; Mittleman, M.L.; Balazs, D. Non-isothermal, in situ XRD analysis of dolomite decomposition. Thermochim. Acta 1989, 140, 67–76.
- Rao, T.R. Kinetic parameters for the decomposition of calcium carbonate. Can. J. Chem. Eng. 1993, 71, 481–484.
- Wang, Y.; Thomson, W.J. The effects of steam and carbon dioxide on calcite decomposition using dynamic X-ray diffraction. Chem. Eng. Sci. 1995, 50, 1373–1382.
- Rao, T.R. Kinetics of calcium carbonate decomposition. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 1996, 19, 373–377.
- Dollimore, D.; Tong, P.; Alexander, K.S. The kinetic interpretation of the decomposition of calcium carbonate by use of relationships other than the Arrhenius equation. Thermochim. Acta 1996, 282, 13–27.
- Kristóf-Makó, É.; Juhász, A.Z. The effect of mechanical treatment on the crystal structure and thermal decomposition of dolomite. Thermochim. Acta 1999, 342, 105–114.
- Dash, S.; Kamruddin, M.; Ajikumar, P.K.; Tyagi, A.K.; Raj, B. Nanocrystalline and metastable phase formation in vacuum thermal decomposition of calcium carbonate. Thermochim. Acta 2000, 363, 129–135.
- Zucchini, A.; Comodi, P.; Nazzareni, S.; Hanfland, M. The effect of cation ordering and temperature on the high-pressure behaviour of dolomite. Phys. Chem. Miner. 2014, 41, 783–793.
- Cai, J.; Wang, S.; Kuang, C. A modified random pore model for carbonation reaction of CaO-based limestone with CO2 in different calcination-carbonation cycles. Energy Procedia 2017, 105, 1924–1931.
- Subagjo; Wulandari, W.; Adinata, P.M.; Fajrin, A. Thermal decomposition of dolomite under CO2-air atmosphere. AIP Conf. Proc. 2017, 1805, 040006.
- Momenzadeh, L.; Moghtaderi, B.; Buzzi, O.; Liu, X.; Sloan, S.W.; Murch, G.E. The thermal conductivity decomposition of calcite calculated by molecular dynamics simulation. Comput. Mater. Sci. 2018, 141, 170–179.
- Zheng, J.; Huang, J.; Tao, L.; Li, Z.; Wang, Q. A Multifaceted Kinetic Model for the Thermal Decomposition of Calcium Carbonate. Crystals 2020, 10, 849.
- Reeder, R.J. Crystal chemistry of the rhombohedral carbonates. Rev. Mineralogy. 1983, 11, 1–47.
- Bater, G. Thermal expansion anisotropy of dolomite-type borates Me2 + Me4 + B2O6. Z. Krist. Cryst. Mater. 1971, 133, 85–90.
- Barber, D.J.; Heard, H.C.; Wenk, H.R. Deformation of dolomite single crystals from 20–800 °C. Phys. Chem. Miner. 1981, 7, 271–286.
- Reeder, R.J.; Markgraf, S.A. High-temperature crystal chemistry of dolomite. Am. Mineral. 1986, 71, 795–804.
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570.
- Markgraf, S.A.; Reeder, R.J. High-temperature structure refinements of calcite and magnesite. Am. Mineral. 1985, 70, 590–600.
- Hazen, R.M.; Prewitt, C.T. Effects of temperature and pressure on interatomic distances in oxygen-based minerals. Am. Mineral. 1977, 62, 309–315.
- Hazen, R.M.; Finger, L.W. Comparative Crystal Chemistry; John Wiley: New York, NY, USA, 1982.
- Sygała, A.; Bukowska, M.; Janoszek, T. High temperature versus geomechanical parameters of selected rocks–the present state of research. J. Sustain. Min. 2013, 12, 45–51.
- Wu, G.; Wang, Y.; Swift, G.; Chen, J. Laboratory investigation of the effects of temperature on the mechanical properties of sandstone. Geotech. Geol. Eng. 2013, 31, 809–816.
- Coppola, M.; Correale, A.; Barberio, M.D.; Billi, A.; Cavallo, A.; Fondriest, M.; Nazzari, M.; Paonita, A.; Romano, C.; Stagno, V.; et al. Meso-to nano-scale evidence of fluid-assisted co-seismic slip along the normal Mt. Morrone Fault, Italy: Implications for earthquake hydrogeochemical precursors. Earth Planet. Sci. Lett. 2021, 568, 117010.
- Zhi-jun, W.; Yang-Sheng, Z.; Yuan, Z.; Chong, W. Research status quo and prospection of mechanical characteristics of rock under high temperature and high pressure. Procedia Earth Planet. Sci. 2009, 1, 565–570.
- Małkowski, P.; Skrzypkowski, K.; Bożęcki, P. Zmiany zachowania się skał pod wpływem wysokich temperatur w rejonie georeaktora. Pr. Nauk. GIG Górnictwo Środowisko 2011, 4, 259–272.
- Małkowski, P.; Kamiński, P.; Skrzypkowski, K. Impact of Heating of carboniferous rocks on their mechanical parameters. AGH J. Min. Geoengin. 2012, 36, 231–242.
- Ranjith, P.G.; Viete, D.R.; Chen, B.J.; Perera, M.S.A. Transformation plasticity and the effect of temperature on the mechanical behavior of Hawkesbury sandstone at atmospheric pressure. Eng. Geol. 2012, 151, 120–127.
- Chen, L.J.; Jun, H.E.; Chao, J.Q.; Qin, B.D. Swelling and breaking characteristics of limestone under high temperatures. Min. Sci. Technol. 2009, 19, 503–507.
- Sivrikaya, O. A study on the physicochemical and thermal characterization of dolomite and limestone samples for use in ironmaking and steelmaking. Ironmak. Steelmak. 2018, 45, 764–772.
- Zhang, W.; Sun, Q. Identification of primary mineral elements and macroscopic parameters in thermal damage process of limestone with canonical correlation analysis. Rock Mech. Rock Eng. 2018, 51, 1287–1292.
- Meng, Q.B.; Wang, C.K.; Liu, J.F.; Zhang, M.W.; Lu, M.M.; Wu, Y. Physical and microstructural characteristics of limestone after high-temperature exposure. Bull. Eng. Geol. Environ. 2020, 79, 1259–1274.
More
