Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
Aortic arch surgery is one of the most complex procedures in cardiac surgery. The gold-standard therapy for the treatment of aortic arch pathologies (including penetrating ulcers, intramural hematoma, aneurysms and pseudoaneurysms) remains open surgery performed under Cardiopulmonary Bypass (CPB) with hypothermic circulatory arrest and selective cerebral perfusion.
- aortic arch stent-grafting
1. Introduction
Nowadays, aortic arch surgery is one of the most complex procedures in cardiac surgery. The gold-standard therapy for the treatment of aortic arch pathologies (including penetrating ulcers, intramural hematoma, aneurysms and pseudoaneurysms) remains open surgery performed under Cardiopulmonary Bypass (CPB) with hypothermic circulatory arrest and selective cerebral perfusion [1].
Starting in the 1990s, hybrid procedures (combination of both open and endovascular procedures) have become an alternative to standard open surgery in order to treat, during the same procedure, both pathologies of the aortic arch and of the descending aorta. The core principle behind this treatment relies on the endovascular exclusion of the pathologic aorta after the creation of an adequate proximal landing zone by means of supra-aortic transposition (debranching) of one, two or three arch vessels. Therefore, the frozen elephant trunk (FET) is considered as the first-choice therapy: this procedure allows the replacement of the aortic arch using a non-stented vascular prosthesis and prepares the thoraco-abdominal aorta for other possible interventions thanks to a distal part composed of a covered stent graft [2][3]. This procedure still requires the use of CPB, circulatory arrest and cerebral perfusion being technically complex with a non-negligible rate of complications, especially in high-risk patients like those who previously had cardiac surgery or the elderly with several comorbidities [4]. For the above-mentioned reasons and in these high-risk patients, total endovascular aortic arch stent-grafting has been proposed as an alternative in selected patients. In these procedures, skin incision is minimal, CPB is not used, cerebral perfusion is always maintained, and they are performed on the beating heart. Therefore, they are by far less invasive than conventional surgery and even less-invasive than what pweople define as minimally invasive surgery (that still requires CPB and cardioplegic arrest). As a consequence, herwe include total endovascular aortic arch exclusion in the so-called micro-invasive (cardiac) surgery procedures [5]. Total endovascular aortic arch stent-grafting can be carried out in different ways: branched devices, fenestrated grafts and using the “chimney technique”.
2. NEXUS Aortic Arch Stent Graft System (Endospan, Herzlia, Israel)
The NEXUS Aortic Arch Stent Graft System is a CE-certified off-the-shelf, single branch, double stent graft system, specifically designed to address aortic pathologies involving or extending in the aortic arch.
It consists of two different components: a main module for the aortic arch and the descending aorta with a side-branch for the brachiocephalic artery (BCA), and a curved module for the ascending aorta that lands into the sino-tubular junction and connects to the main module through a side-facing self-protecting sleeve (Figure 1).
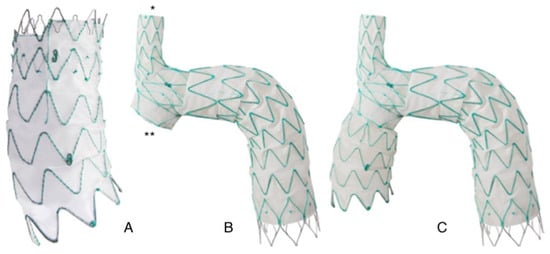

Figure 1. Nexus aortic arch system: (A): Ascending module. The module its pre-curved to adapt to the curvature of the ascending aorta. (B): Main module. The module features an integrated side branch (*) for the brachio-cephalic artery and a self-projecting sleeve (**) that faces the ascending aorta and allows connection with the ascending module. (C): Finally assembled device. The two modules are connected through an interlocking system that provides strong separation force reducing the risk of disconnection and of type 3 endoleak.
Although BCA is the suggested approach for main module deployment, landing is possible in any suitable supra-aortic vessel after careful evaluation of its anatomy. Generally, the left subclavian artery represents a good alternative landing site (Figure 2).
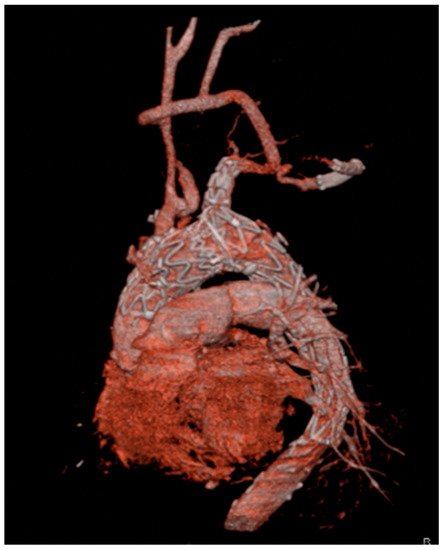

Figure 2. Three-dimensional reconstruction of a Nexus device with the side-branch positioned in the left subclavian artery. Patent and well-functioning supra-aortic debranching is clearly visible.
Tantalum radiopaque markers and an active locking mechanism facilitate the correct rotational and longitudinal deployment in order to achieve good sealing and to avoid coverage of the BCA branch. Like other off-the-shelf devices, different sizes of the components are available to fit the great majority of aortic anatomies.
Before device implantation, an extra anatomical surgical debranching of supra-aortic vessels is mandatory to redirect blood to the brain and to upper arms vessel from the BCA. To minimize handling and arch friction, the delivery systems are hydrophilic and pre-shaped. The delivery system is compatible with a 20 Fr introducer. The procedure is performed under general anesthesia and fluoroscopy guidance with bilateral percutaneous femoral and right axillary artery access (or left if the target vessel is the left subclavian artery). Near-infrared spectroscopy (NIRS) may be used as a cerebral monitoring system to rule out significant perfusion deficit during the procedure. A through-and-through axillary-femoral artery guideline is placed. A temporary trans-venous pacemaker lead is placed in the right ventricle for rapid pacing during the stent deployment. After systemic heparinization (ACT > 350 s), the main module is advanced over the guidewire to its final position and deployed from the BCA to the descending aorta. Subsequently, via the stiff guidewire placed in the left ventricle and during rapid pacing the ascending aorta module is implanted and connected to the main module through the self-docking sleeve and the delivery system is then withdrawn. Care has to be taken in positioning the ascending module at the level of the tantalum ring of the main module to achieve adequate fixation and sealing. Under rapid pacing, a simultaneous inflation of two molding balloons one at the locking section and the second at the BCA branch, is performed.
A multicenter study involving five centers in Europe, Canada and New Zealand investigated safety and performance of NEXUS. Twenty-eight patients (mean age 72 ± 6 years) with aortic arch pathology considered to be at high or prohibitive risk for surgery and with anatomical fit criteria for endoprosthesis implantation were enrolled [6]. Most of them were affected by an isolated arch aneurysm (61%) and chronic aortic dissection (21%). Exclusion criteria were acute dissections, suspected infectious etiology and connective tissue disease. All patients underwent a successful implantation of the device without needing for conversion to open surgery or intraoperative death. At 30 days, successful treatment of the disease was 96%, while freedom from procedure-related mortality was 93% (two patients died: one suddenly and one due to multiple brain infarcts). No device-related mortality was recorded at 30 days. Among major adverse events, only one patient developed an acute kidney injury requiring dialysis, while paraplegia, disabling stroke, ruptured aneurysm, myocardial infarction and new onset of aortic regurgitation were not observed. One year later, freedom from unplanned device related reoperation was 89%. There was no evidence of graft migration, prosthesis separation, stent fracture, branch occlusion, graft infolding or collapse. One more death, not device-related, was registered; one patient had a transient ischemic event. There were no additional reports of renal injury, paraplegia, myocardial infarction and aortic regurgitation.
3. RelayBranch Thoracic Stent-Graft System (Terumo Aortic, Glasgow, United Kingdom)
The RelayBranch Thoracic Stent-Graft System is a custom made, double branched endograft with a wide window on its superior portion to accommodate two inner tunnels for BCA and left common carotid artery (LCCA) connection (Figure 3 and Figure 4).

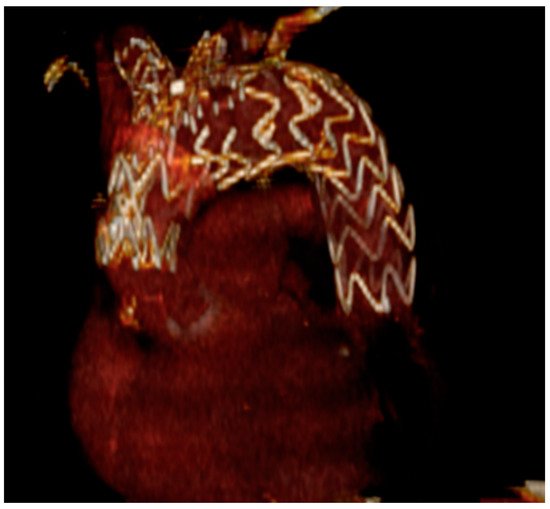

Figure 3. Relaybranch aortic arch system. The figure shows the stent-graft with two side-branches (for the brachio-cephalic trunk and for the left common carotid artery) that are positioned in the two tunnels of the upper window.

Figure 4. Three-dimensional reconstruction of total endovascular aortic arch exclusion with the RelayBranch device.
These extensions are custom-made or off-the-shelf bridging limbs. Stent grafts are manufactured according to preoperative CT scan measurement and the customization process generally takes about 4 to 8 weeks. A pre-curved delivery system allows a window for supra-aortic branches to be automatically aligned with the outer curvature of the aorta. Radiopaque markers are placed in the window and in the ends of the stent grafts to facilitate the positioning and the reorientation. Specific lock stent systems are present in the tunnels to prevent potential disconnection and migration of the extensions. A left subclavian artery (LSA) revascularization is needed prior to the procedure.
The procedure is carried out under general anesthesia, angiography guidance, NIRS monitoring and systemic heparinization (ACT > 350 s) as described above for a single-branch stent graft system. Usually, a common femoral artery access is used to place a stiff guidewire into the left ventricle and to advance the main stent graft in the aortic arch. Rapid pacing through a temporary trans-venous pacemaker is established while the endoprosthesis is deployed. Bilateral cervical accesses are generally used to advance soft guidewires for catheterization of the inner tunnels in a retrograde fashion. Once the target tunnel is engaged, a stiffer wire is introduced, and the correct positioning is monitored under fluoroscopy. Then the extension graft is deployed with maximum overlapping within the tunnel and inflation of a molding balloon is performed to ensure a correct sealing of the components. The same procedure is done for the other tunnel in a sequential fashion.
The Italian registry of double inner branch stent graft for arch pathology (TRIUMPH Registry) evaluates early and mid-term results in 24 patients, from nine Italian cardiovascular centers, considered unfit for open arch surgery and treated with the RelayBranch Thoracic Stent-Graft System [7]. All patients were male (75 ± 7 years), mostly affected by atherosclerotic aneurysm (54%) and penetrating aortic ulcer (38%); 29% of them underwent a previous open aortic repair. The in-hospital mortality rate was 16.7% and cerebral events occurred in six patients (25%) with three major strokes. Two patients experienced a retrograde dissection (8.3%) and no type I or II endoleak was reported. During a mean follow up of 18 months, only one death and one more non-arch-related major stroke were registered; no secondary intervention was needed and no new onset of type I or III endoleak, branch occlusion, disconnection or migration were noted.
A Japanese cohort of 28 patients (mean age 78 ± 7 years, 61% males, mean Logistic EUROscore 34%) treated with a double branched endograft reported a complete procedural success without any in hospital death [8]. Four patients (14%) had symptomatic strokes and two of these were disabling. Cumulative survival rate and aorta-related death-free were, respectively, 81% and 96%. During follow up, five total deaths occurred, including one aorta-related death due to aneurysmal rupture secondary to a type Ib endoleak; another patient experienced a type 3 endoleak, successfully treated with an additional TEVAR.
References
- Lou, X.; Chen, E.P. Optimal Cerebral Protection Strategies in Aortic Surgery. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 146–152.
- Di Marco, L.; Pantaleo, A.; Leone, A.; Murana, G.; Di Bartolomeo, R.; Pacini, D. The Frozen Elephant Trunk Technique: European Association for Cardio-Thoracic Surgery Position and Bologna Experience. Korean J. Thorac. Cardiovasc. Surg. 2017, 50, 1–7.
- Vendramin, I.; Lechiancole, A.; Frigatti, P.; Sponza, M.; Sponga, S.; Piani, D.; Bortolotti, U.; Livi, U. Aortic arch aneurysm and Kommerell’s diverticulum: Repair with a single-stage hybrid approach. J. Card. Surg. 2019, 34, 641–644.
- Martens, A.; Beckmann, E.; Kaufeld, T.; Umminger, J.; Fleissner, F.; Koigeldiyev, N.; Shrestha, M. Total aortic arch repair: Risk factor analysis and follow-up in 199 patients. Eur. J. Cardio-Thorac. Surg. 2016, 50, 940–948.
- D’Onofrio, A.; Gerosa, G. Shifting a Paradigm of Cardiac Surgery: From Minimally Invasive to Micro-Invasive. J. Heart Valve Dis. 2015, 24, 528–530.
- Planer, D.; Elbaz-Greener, G.; Mangialardi, N.; Lindsay, T.; D’Onofrio, A.; Schelzig, H.; Chaykovska, L.; Hill, A.; Holden, A.; Antonello, M.; et al. NEXUS Arch: A Multicenter Study Evaluating the Initial Experience with a Novel Aortic Arch Stent Graft System. Ann. Surgery 2021.
- Ferrer, C.; Cao, P.; Coscarella, C.; Ferri, M.; Lovato, L.; Camparini, S.; di Marzo, L. TRIUmPH Registry Investigators. iTalian RegIstry of doUble inner branch stent graft for arch PatHology (the TRIUmPH Registry). J. Vasc. Surg. 2019, 70, 672–682.e1.
- Kudo, T.; Kuratani, T.; Shimamura, K.; Sawa, Y. Early and midterm results of thoracic endovascular aortic repair using a branched endograft for aortic arch pathologies: A retrospective single-center study. JTCVS Tech. 2020, 26, 17–25.
More
