Fumonisins are widely found in animal feed, feed raw materials, and human food. This can not only cause economic losses in animal husbandry but can also have carcinogenicity or teratogenicity and can be left in animal meat, eggs, and milk which may enter the human body and pose a serious threat to human health. Fumonisins cause a variety of toxic effects to organisms including autophagy, apoptosis, neurotoxicity, immunotoxicity, reproductive toxicity, tissue and organ toxicity, and carcinogenicity. They can not only cause disease alone but also have a combined toxic effect with other mycotoxins such as aflatoxins. The toxicity of fumonisins is a very complex process. Previous studies report that fumonisins exert their toxicity by modulating sphingolipid metabolism and inducing oxidative stress
- Mechanism
- Fumonisins
- Oxidative Stress
1. Introduction
2. Effect of Fumonisins on Sphingolipid Synthesis
3. Fumonism Induces Oxidative Stress
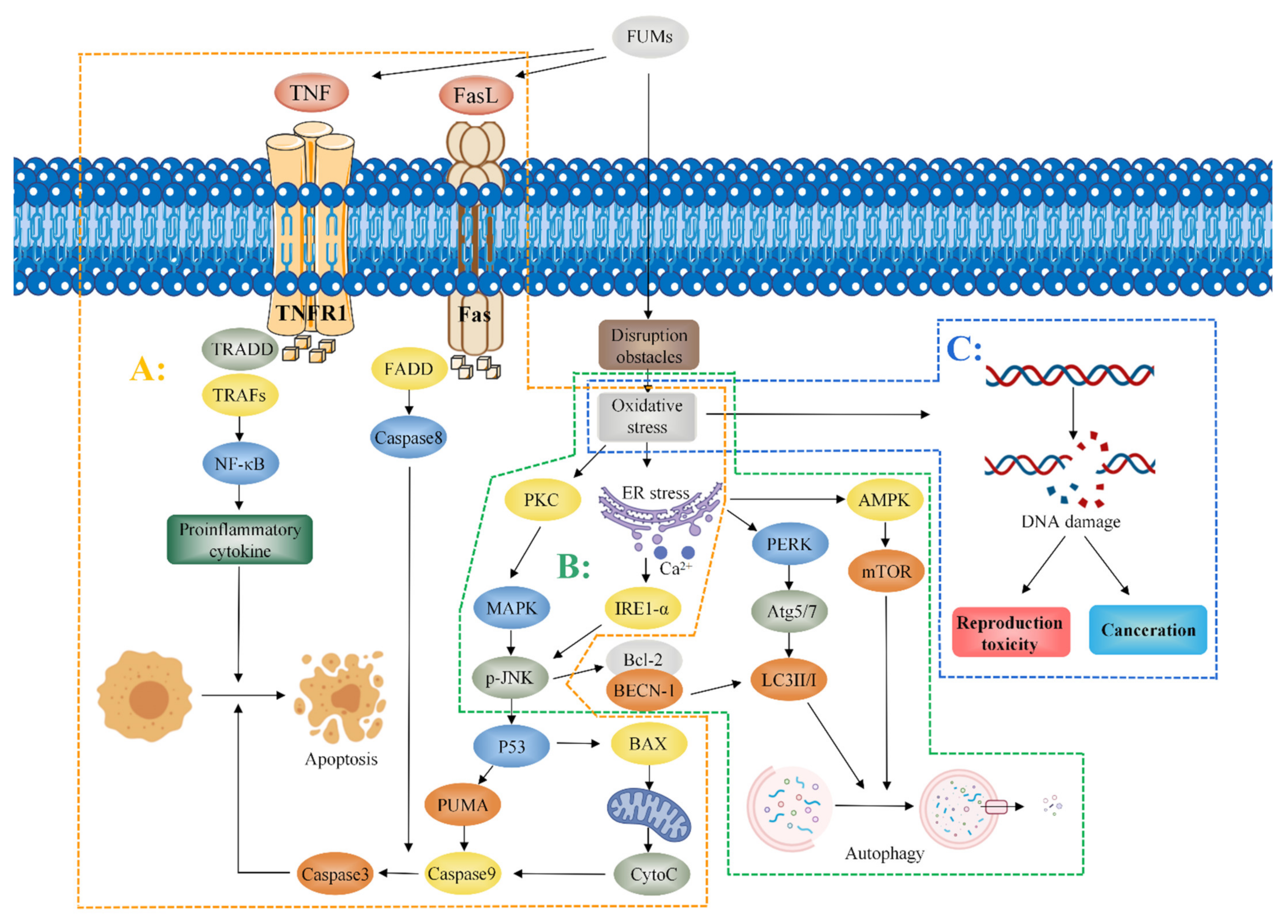
References
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. IJERPH 2017, 14, 632.
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279.
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.-S. Co-Contamination of Aflatoxin B1 and Fumonisin B1 in Food and Human Dietary Exposure in Three Areas of China. Food Addit. Contam. Part A 2011, 28, 461–470.
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033.
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin Distribution in Maize Dry-Milling Products and By-Products: Impact of Two Industrial Degermination Systems. Toxins 2018, 10, 357.
- Yang, C.; Song, G.; Lim, W. Effects of Mycotoxin-Contaminated Feed on Farm Animals. J. Hazard. Mater. 2020, 389, 122087.
- Gelineau-van Waes, J.; Voss, K.A.; Stevens, V.L.; Speer, M.C.; Riley, R.T. Chapter 5 Maternal Fumonisin Exposure as a Risk Factor for Neural Tube Defects. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 56, pp. 145–181. ISBN 978-0-12-374439-5.
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372.
- Li, R.; Tao, B.; Pang, M.; Liu, Y.; Dong, J. Natural Occurrence of Fumonisins B1 and B2 in Maize from Three Main Maize-Producing Provinces in China. Food Control 2015, 50, 838–842.
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by Fusarium Species. Appl Env. Microbiol 2002, 68, 2101–2105.
- Chen, J.; Li, Z.; Cheng, Y.; Gao, C.; Guo, L.; Wang, T.; Xu, J. Sphinganine-Analog Mycotoxins (SAMs): Chemical Structures, Bioactivities, and Genetic Controls. J. Fungi 2020, 6, 312.
- World Health Organization (WHO). Fumonisins. In Safety Evaluation of Certain Mycotoxins in Food; WHO Food Additives Series 47; FAO Food and Nutrition Paper 74; WHO: Geneva, Switzerland, 2001.
- European Commission Regulation No. 1881/2006, setting maximum levels for certain contaminants in foodstuffs, 19 December 2006. Off. J. Eur. Union 2006, L364, 5–24.
- European Commission Regulation No. 1126/2007, setting maximum levels for certain contaminants in foodstuffs, 28 September 2007. Off. J. Eur. Union 2007, L255, 14–17.
- International Agency for Research on Cancer. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; This Publication Represents the Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Which Met in Lyon, 12–19 February 2002; IARC monographs on the evaluation of carcinogenic risks to humans; IARC: Lyon, France, 2002; ISBN 978-92-832-1282-9.
- Bennett, J.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516.
- Zhu, Y.; Hassan, Y.; Lepp, D.; Shao, S.; Zhou, T. Strategies and Methodologies for Developing Microbial Detoxification Systems to Mitigate Mycotoxins. Toxins 2017, 9, 130.
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent Trends in Detecting, Controlling, and Detoxifying of Patulin Mycotoxin Using Biotechnology Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472.
- Schertz, H.; Dänicke, S.; Frahm, J.; Schatzmayr, D.; Dohnal, I.; Bichl, G.; Schwartz-Zimmermann, H.; Colicchia, S.; Breves, G.; Teifke, J.; et al. Biomarker Evaluation and Toxic Effects of an Acute Oral and Systemic Fumonisin Exposure of Pigs with a Special Focus on Dietary Fumonisin Esterase Supplementation. Toxins 2018, 10, 296.
- Qin, X.; Zhang, R.-X.; Ge, S.; Zhou, T.; Liang, Y.-K. Sphingosine Kinase AtSPHK1 Functions in Fumonisin B1-Triggered Cell Death in Arabidopsis. Plant Physiol. Biochem. 2017, 119, 70–80.
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191.
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50.
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of Sphingolipids in Senescence: Implication in Aging and Age-Related Diseases. J. Clin. Investig. 2018, 128, 2702–2712.
- Riley, R.T.; Merrill, A.H. Ceramide Synthase Inhibition by Fumonisins: A Perfect Storm of Perturbed Sphingolipid Metabolism, Signaling, and Disease. J. Lipid Res. 2019, 60, 1183–1189.
- Gelineau-van Waes, J.; Rainey, M.A.; Maddox, J.R.; Voss, K.A.; Sachs, A.J.; Gardner, N.M.; Wilberding, J.D.; Riley, R.T. Increased Sphingoid Base-1-Phosphates and Failure of Neural Tube Closure after Exposure to Fumonisin or FTY720. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 790–803.
- Gardner, N.M.; Riley, R.T.; Showker, J.L.; Voss, K.A.; Sachs, A.J.; Maddox, J.R.; Gelineau-van Waes, J.B. Elevated Nuclear Sphingoid Base-1-Phosphates and Decreased Histone Deacetylase Activity after Fumonisin B1 Treatment in Mouse Embryonic Fibroblasts. Toxicol. Appl. Pharmacol. 2016, 298, 56–65.
- Kim, D.-H.; Yoo, H.-S.; Lee, Y.-M.; Kie, J.-H.; Jang, S.; Oh, S. Elevation of Sphinganine 1-Phosphate as a Predictive Biomarker for Fumonisin Exposure and Toxicity in Mice. J. Toxicol. Environ. Health Part A 2006, 69, 2071–2082.
- Voss, K.A.; Howard, P.C.; Riley, R.T.; Sharma, R.P.; Bucci, T.J.; Lorentzen, R.J. Carcinogenicity and Mechanism of Action of Fumonisin B1: A Mycotoxin Produced by Fusarium Moniliforme (=F. Verticillioides). Cancer Detect. Prev. 2002, 26, 1–9.
- Enongene, E.N. Persistence and Reversibility of the Elevation in Free Sphingoid Bases Induced by Fumonisin Inhibition of Ceramide Synthase. Toxicol. Sci. 2002, 67, 173–181.
- Grenier, B.; Schwartz-Zimmermann, H.; Caha, S.; Moll, W.; Schatzmayr, G.; Applegate, T. Dose-Dependent Effects on Sphingoid Bases and Cytokines in Chickens Fed Diets Prepared with Fusarium Verticillioides Culture Material Containing Fumonisins. Toxins 2015, 7, 1253–1272.
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic Ingestion of Deoxynivalenol and Fumonisin, Alone or in Interaction, Induces Morphological and Immunological Changes in the Intestine of Piglets. Br. J. Nutr. 2012, 107, 1776–1786.
- Atroshi, F.; Rizzo, A.; Biese, I.; Veijalainen, P.; Saloniemi, H.; Sankari, S.; Andersson, K. Fumonisin B1-Introduce DNA Damage in Liver and Spleen: Effects of Pretreatment Wuth Coenzyme. Pharmacol. Res. 1999, 40, 9.
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Anadón, A.; Yuan, Z. Fumonisins: Oxidative Stress-Mediated Toxicity and Metabolism in Vivo and in Vitro. Arch. Toxicol. 2016, 90, 81–101.
- Sheik Abdul, N.; Marnewick, J.L. Fumonisin B1-induced Mitochondrial Toxicity and Hepatoprotective Potential of Rooibos: An Update. J. Appl. Toxicol. 2020, 40, 1602–1613.
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Oxidative Stress Induced by Fumonisin B1 in Continuous Human and Rodent Neural Cell Cultures. Free Radic. Res. 2004, 38, 933–942.
- Arumugam, T.; Pillay, Y.; Ghazi, T.; Nagiah, S.; Abdul, N.S.; Chuturgoon, A.A. Fumonisin B1-Induced Oxidative Stress Triggers Nrf2-Mediated Antioxidant Response in Human Hepatocellular Carcinoma (HepG2) Cells. Mycotoxin Res. 2019, 35, 99–109.
- Singh, M.P.; Kang, S.C. Endoplasmic Reticulum Stress-Mediated Autophagy Activation Attenuates Fumonisin B1 Induced Hepatotoxicity in Vitro and in Vivo. Food Chem. Toxicol. 2017, 110, 371–382.
- Kim, S.H.; Singh, M.P.; Sharma, C.; Kang, S.C. Fumonisin B1 Actuates Oxidative Stress-Associated Colonic Damage via Apoptosis and Autophagy Activation in Murine Model. J. Biochem. Mol. Toxicol. 2018, 32, e22161.
- Domijan, A.; Želježić, D.; Peraica, M.; Kovačević, G.; Gregorović, G.; Krstanac, Ž.; Horvatin, K.; Kalafatić, M. Early Toxic Effects of Fumonisin B1 in Rat Liver. Hum. Exp. Toxicol. 2008, 27, 895–900.
- Minervini, F.; Lacalandra, G.M.; Filannino, A.; Garbetta, A.; Nicassio, M.; Dell’Aquila, M.E.; Visconti, A. Toxic Effects Induced by Mycotoxin Fumonisin B1 on Equine Spermatozoa: Assessment of Viability, Sperm Chromatin Structure Stability, ROS Production and Motility. Toxicol. Vitr. 2010, 24, 2072–2078.
- Mobio, T.A.; Tavan, E.; Baudrimont, I.; Anane, R.; Carratú, M.-R.; Sanni, A.; Gbeassor, M.F.; Shier, T.W.; Narbonne, J.-F.; Creppy, E.E. Comparative Study of the Toxic Effects of Fumonisin B1 in Rat C6 Glioma Cells and P53-Null Mouse Embryo Fibroblasts. Toxicology 2003, 183, 65–75.
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139.
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2′-Deoxyguanosine as a Marker of Oxidative DNA Damage Related to Occupational and Environmental Exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15.
- Yuan, Q.; Jiang, Y.; Fan, Y.; Ma, Y.; Lei, H.; Su, J. Fumonisin B1 Induces Oxidative Stress and Breaks Barrier Functions in Pig Iliac Endothelium Cells. Toxins 2019, 11, 387.
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Zhang, S.; Wu, A. Fumonisin B1 Triggers Carcinogenesis via HDAC/PI3K/Akt Signalling Pathway in Human Esophageal Epithelial Cells. Sci. Total Environ. 2021, 787, 147405.
- Gopee, N.V.; He, Q.; Sharma, R.P. Fumonisin B1-Induced Apoptosis Is Associated with Delayed Inhibition of Protein Kinase C, Nuclear Factor-ΚB and Tumor Necrosis Factor α in LLC-PK1 Cells. Chem. Biol. Interact. 2003, 146, 131–145.
- Li, H.; Wang, M.; Kang, W.; Lin, Z.; Gan, F.; Huang, K. Non-Cytotoxic Dosage of Fumonisin B1 Aggravates Ochratoxin A-Induced Nephrocytotoxicity and Apoptosis via ROS-Dependent JNK/MAPK Signaling Pathway. Toxicology 2021, 457, 152802.
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870.
- Khan, R.; Phulukdaree, A.; Chuturgoon, A. Concentration-Dependent Effect of Fumonisin B1 on Apoptosis in Oesophageal Cancer Cells. Hum. Exp. Toxicol. 2018, 37, 762–771.
- Bucciantini, M.; Nosi, D.; Forzan, M.; Russo, E.; Calamai, M.; Pieri, L.; Formigli, L.; Quercioli, F.; Soria, S.; Pavone, F.; et al. Toxic Effects of Amyloid Fibrils on Cell Membranes: The Importance of Ganglioside GM1. FASEB J. 2012, 26, 818–831.
- Du, P.; Li, S.-J.; Ojcius, D.M.; Li, K.-X.; Hu, W.-L.; Lin, X.; Sun, A.-H.; Yan, J. A Novel Fas-Binding Outer Membrane Protein and Lipopolysaccharide of Leptospira Interrogans Induce Macrophage Apoptosis through the Fas/FasL-Caspase-8/-3 Pathway. Emerg. Microbes Infect. 2018, 7, 135.
- He, Q.; Bhandari, N.; Sharma, R.P. Fumonisin B1 Alters Sphingolipid Metabolism and Tumor Necrosis Factor a Expression in Heart and Lung of Mice. Life Sci. 2002, 9, 2015–2023.
- Kócsó, D.J.; Szabó-Fodor, J.; Mézes, M.; Balogh, K.; Ferenczi, S.; Szabó, A.; Bóta, B.; Kovács, M. Fumonisin B1 Exposure Increases Hsp70 Expression in the Lung and Kidney of Rats without Inducing Significant Oxidative Stress. Acta Vet. Hung. 2018, 66, 394–407.
- Chen, J.; Yang, S.; Huang, S.; Yan, R.; Wang, M.; Chen, S.; Cai, J.; Long, M.; Li, P. Transcriptome Study Reveals Apoptosis of Porcine Kidney Cells Induced by Fumonisin B1 via TNF Signalling Pathway. Food Chem. Toxicol. 2020, 139, 111274.
- Régnier, M.; Gourbeyre, P.; Pinton, P.; Napper, S.; Laffite, J.; Cossalter, A.; Bailly, J.; Lippi, Y.; Bertrand-Michel, J.; Bracarense, A.P.F.R.L.; et al. Identification of Signaling Pathways Targeted by the Food Contaminant FB1: Transcriptome and Kinome Analysis of Samples from Pig Liver and Intestine. Mol. Nutr. Food Res. 2017, 61, 1700433.
