Fuel cells (FCs) provide electricity via the generation of ion carriers by electrocatalysis at the electrodes as well as a positive or negative ion transport mechanism and direction of motion through electrolyte membranes. Pt nanomaterials are used in the catalytic layer components of low-temperature FCs associated with the clean H2 fuel industry, which are the most successful and typical examples of generating clean electric energy and power.
- Pt nanocatalysts
- Low Temperature Fuel Cells
- The sol-gel process
- The polyol process
- The combined sol-gel and polyol processes
- Electronics
- Photonics
- Optoelectronics
- Energy & environment
- Electronics and telecommunication
1. Introduction
At present, fuel cells (FCs), proton exchange membrane fuel cells (PEMFCs), and direct methanol FCs (DMFCs) using excellent Pt electrocatalysts have played an increasing role for engineering, science, technology, and industry [1][2][1,2]. An FC provides electricity via the generation of ion carriers by electrocatalysis at the electrodes as well as a positive or negative ion transport mechanism and direction of motion through electrolyte membranes. In many recent years, modified polyol methods have played an important role in the controlled synthesis of various kinds of crystal nanoparticles used as the nanostructured catalysts applied in energy and environment [3][4][5][6][7][8][9][3,4,5,6,7,8,9]. An FC is a power generation system used to produce electricity using hydrogen fuel with an electrode membrane assembly, which is considered an ion conductor. The electrocatalyst layer involved in the purely so-called standard Pt nanocatalyst, or the special nanocatalyst layer was equivalent relatively to Pt nanocatalyst standard [10][11][12][13][14][15][16][17][10,11,12,13,14,15,16,17]. It is explained that their catalytic and electrocatalytic characterizations originated from high surface-to-volume ratio and quantum size [3]. The various types of Pt-based, Pd-based, Pd-free, Pt-free multimetal nanocatalysts have been being studied as promising candidates to replace the standard Pt catalyst because of its very high cost for low temperature FCs. Here, Pt-group metals (PGM) consist of Ru, Rh, Pd, Os, Ir, and Pt, which means that Pt-M bimetal catalysts for FCs can be synthesized by modified polyol methods. It is known that Pt electrocatalysts are widely used for studying hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and oxygen evolution reaction (OER) processes in cyclic voltammogram (CV) cycles. In the key points, ORR/OER and HER/OER of Pt- and Pd-based alloy and core-shell nanoparticles electrocatalysts are crucial in order to improve catalytic materials for low temperature FCs. The most important advantages of Pt-based core-shell nanoparticles are applied for reducing the high cost of FCs, DMFCs, and PEMFCs using Nafion® membranes or the hydrophobic perfluorocarbon backbone of -(CF2)n-groups and the chains (–SO3H) [1][2][1,2]. In various works, Ni-, Co-, and Fe-based oxide micro/nanosized particles with grain and grain boundaries were prepared because they showed high structural durability and stability [18][19][20][21][22][23][18,19,20,21,22,23]. In particular significance, they can be used as the oxide supports for noble metal and multimetal nanocatalysts. They are very promising candidates for FCs, DMFCs, PEMFCs, and high-temperature solid oxide FCs (SOFCs) as well as batteries and capacitors [1][2][1,2]. However, the inexpensive cost and long lifetime of PEMFCs and DMFCs are very importantly required [10][11][12][13][14][15][16][17][10,11,12,13,14,15,16,17].
2. Pt Nanocatalyst in Low Temperature FCs
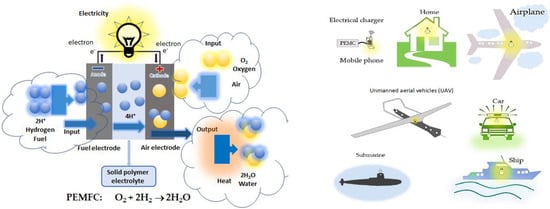
| FCs | Input/Output | Anode | Electrolyte | Ion | Cathode |
|---|---|---|---|---|---|
| PEMFC WT: 60–120°C H+ transport |
In: H2, O2 (Air) Out: H2O, heat, extra gases |
H2 → 2H+ + 2e− Pt catalyst |
Membrane H+ → Membrane → Cathode |
H+ | ½O2 + 2H+ + 2e− → H2O; Pt catalyst; Air as oxidant |
| DMFC WT: 60–120 °C H+ transport |
In: CH3OH, O2 (Air) Out: CO2, H2O, heat, extra gases |
CH3OH + H2O → CO2 + 6H+ + 6e− Pt catalyst |
Membrane H+ → Membrane → Cathode |
H+ | 3/2O2 + 6H+ + 6e− → 3H2O; Pt catalyst; Air as oxidant |
| AFC WT < 100 °C OH− transport |
In: H2, O2 (Air) Out: H2O |
H2 + 2OH− → 2H2O + 2e− Electrode material |
KOH Anode ← KOH ← OH− |
OH− | ½O2 + 2H2O + 2e− → 2OH− Electrode material |
| PAFC WT: 160–220°C H+ transport |
In: H2, O2 (Air) Out: H2O, heat, extra gases |
H2 → 2H+ + 2e− Pt catalyst |
H3PO4 H+ → H3PO4 → Cathode |
H+ | ½O2 + 2H+ + 2e− → H2O; Pt catalyst; Air as oxidant |
| MCFC WT: 600–800°C CO32− transport |
In: CHx, CO, H2 Out: H2O, heat, extra gases |
H2 + CO32− → 2H2O + 2e− Electrode material |
Molten carbonate Anode ← Molten carbonate ← CO32− |
CO32− | ½O2 + CO2 + 2e− → CO32−; Electrode material; Air as oxidant |
| SOFC WT: 800–1000°C O2− transport |
In: CHx, CO, H2 Out: H2O, heat, extra gases |
H2 + O2− → H2O + 2e− Electrode material |
Ceramics Anode ← Ceramics ← O2− |
O2− | ½O2 + 2e− → O−2 Electrode material; Air as oxidant |
| DCFC WT: 500–1000°C O2− transport |
In: Carbon, CO Out: CO, CO2, heat, extra gases |
C + 2CO32− → 3CO2 + 4e− Electrode material |
Ceramics Anode ← Ceramics ← O2− |
O2− | C + CO2 → 2CO Electrode material; Air as oxidant |
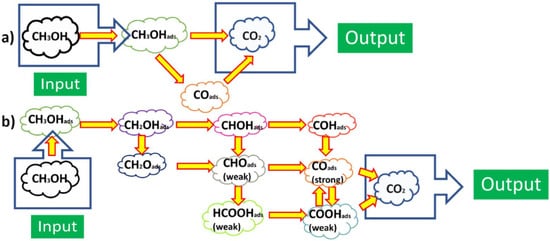
For the use of hydrogen fuel, ORR at the cathode: ½O2+2H++2e−→H2O, hydrogen oxidation reaction (HOR) at the anode: H2→2H++2e−, and the whole reaction of PEMFC using H2: ½O2+H2→ H2O or O2+2H2→2H2O. For DMFCs, under the effect of electronic catalytic layer on the basis of Pt electrocatalyst on the electrodes, the principle of its operation is based on the chemical reaction on the electrode surface. In acidic solutions, the catalytic processes and mechanisms occurred at the electrode surface of the Pt-based electrocatalysts exhibiting the seven specific regions of HOR and ORR in CVs in the kinetics of electrochemical reactions [1][2][1,2], and information [24]and information reviewed [24]: (1) Pt−Hads→Pt+H++e−; (2) QDL(Charge)↔QDL(Discharge); (3) Pt+H2O→Pt−OH+H++e−; (4) PtOH+H2O→Pt(OH)2+H++e−; (5) Pt−(OH)2→PtO+H2O; (6) 2PtO+4H++4e−→Pt−Pt+2H2O; (7) Pt+H++e−→Pt−Hads. For methanol fuel, ORR at the cathode as 3/2O2+6H++6e−→3H2O, methanol oxidation reaction (MOR) at the anode: CH3OH+H2O→CO2+6H++6e−, and the whole reaction of DMFC as CH3OH+3/2O2→ CO2+2H2O [24]. In the most effective MOR in acidic solutions, researchers show that electrocatalytic activity of Pt catalysts to methanol oxidation occurs at (111), (110), (100) and (hkl) low-index crystal planes of Pt nanoparticles as follows: (1) Pt+CH3OH→Pt−(COH)ads+3H++3e−; (2) Pt−(COH)ads+H2O→Pt+CO2+3H++3e−. The catalytic mechanisms of both ORR and MOR are also presented in Scheme 1, which only leads to show CH3OH oxidation into CO2 experimentally. In recent years, PtRu-based electrocatalytic bimetal nanomaterials have been studied in the effective reduction of CO poisoning by Ru according to bifunctional catalytic mechanism, i.e., Ru+H2O→Ru−OH+H++e−, and Ru−OH+Pt−CO→Pt+Ru+CO2+H++e−.
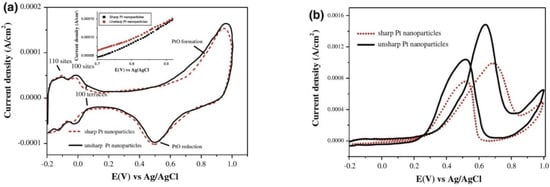
However, the cost of Pd and Ru is relatively high to PtRu electrocatalyst. We need to select other inexpensive, non-noble, non-rare metals, such as Au, Ag, Cu, Fe, Co, Ni, Sn, Mo, Pb, W, etc., rather than Pt metal group (PMG) bimetal catalysts, such as PtPd, PtRh, PtRu, PtIr, PtOs [11][12][13] [11,12,13]. There have been published works related to the synthesis of single metal Pt and bimetallic Pt-based nanomaterial catalysts so that they are elements of PMG, such as Ru, Rh, Pd, Ir, and Os, but their cost is high. There are Pt-based nanoparticles with other inexpensive metals, such as Cu, Ni, Co, and Fe, and their Pt-based or Pt-free multi-component, alloy, multimetal electrocatalysts by improved polyol processes with a strong reducing agent (NaBH4 or KBH4) [13] or other strong reducing solid compounds (CaH2) or reducing gases (H2) in heat treatment [24]reviewed [24]. In future, the application of the improved polyol method is suitable for all the popular laboratories. This is a chemical process popular in laboratory that can be easily applied to create electrocatalytic Pt-based nanomaterials, which is a very necessary composite material in the electrocatalytic layers of PEMFCs and DMFCs today, with increasingly scientific and practical significance. Therefore, the polyol process is one of the focuses discussed in order to address the synthesis of single metal, bimetal, and multimetal nanoparticles, especially for shell-core bimetallic nanostructures. In the synthesis of metal, oxide, and alloy nanostructures, especially instead of using inexpensive precious metals, bimetallic alloys, multimetal alloys, or multi-component materials for catalyst of FCs, and magnetic nanoparticles for practical applications in medicine and biology, issues of size, shape, structure and composition are of great importance. Therefore, these parameters must be studied and controlled. In order to confirm that Pt-based nanocatalyst materials can be applied to FCs (PEMFCs and DMFCs), such nanomaterials must be intensively studied for the electrochemical properties of the used catalytic materials on the surfaces of the electrodes. The important electrochemical reactions of using oxygen, methanol, ethanol, or other fuels are ORR, MOR, and ethanol oxidation reaction (EOR) [10][11][12][13][14][10,11,12,13,14]. It is certain that Pt-based catalysts are used in the anode and cathode of low-temperature fuel cell systems. For catalytic applications, other nanoparticles (Au, Ag, Cu, and their related oxides), iron oxide particles (iron and their compounds), spinel oxide particles, and ABO3-type perovskite oxide particles could potentially be used in the future [1][2][11][12][13][14][15][16][17][1,2,11,12,13,14,15,16,17]. In addition, multi-component, multi-metallic particle catalysts, or functional catalytic oxide particles need to be studied with regard to their practical applications and commercialized products [16]. Scientific research methodology and theoretical and experimental research methods of other nanomaterials are applied as for the special case of Pt nanoparticle materials. The polyol method is a good solution for the comprehensive fabrication of platinum nanoparticles. Over the past ten years, there have been the intensive studies on the successful synthesis of Pt-based catalysts by polyol method by researchers in laboratories which have been presented, reported and published. Therefore, it is believed that the nanomaterials capable of replacing Pt catalysts, such as bimetal catalysts, i.e., PtCu, PtAg, PtAu, PtFe, PtNi, PtCo, and other catalytic alloys that are much cheaper in order to replace expensive Pt that can be used for applications of low-temperature FCs [13]. The two kinds of FePt and CoPt magnetic nanomaterials have been also used in hard disk drives. The deep discussion of research results on the successful synthesis of Pt nanoparticles by nanochemistry has been carried out on published works, typically for modified polyol methods or nanochemistry [3][4][5][6][7][8][9][3,4,5,6,7,8,9]. It is known that Pt nanoparticles have been successfully fabricated by the chemical methods. Given the scientific implications of current research on Pt nanoparticles, precious metals, inexpensive metals, oxide materials, and alloys nanoparticles are very necessary to be mainly focused on their structures and properties. Up to the present time in 2021, Pt nanoparticles, and Pt-based shell-core nanoparticles have been applied in energy technologies, typically such as FC technology, allowing the fabrication of creating mobile phones, transport vehicles, and clean energy sources for households in remote places. Many energy projects have mainly focused on Pt nanomaterials as well as PGM-free catalysts and alternative electrocatalysts [1][2][16][17][1,2,16,17]. In a number of present studies, it is possible to synthesize Pt nanoparticles in the range of 10 nm, and Pt-based bimetallic nanoparticles in the range of 30 nm or up to hundreds of nm in size. Thus, the successful synthesis of metal, bimetal, and multimetal nanoparticles has very high scientific and practical significance for potential application in new technologies of electronic catalysis, photocatalysis, energy, medicine, and biology [24]that were reviewed [24]. At present, a large number of Pt single-metal nanostructures are also researched and developed by chemical methods. Through the polyol process, scientists have successfully fabricated Pt simple-metal nanoparticles for catalysis, but Cu, Au, and Ag nanoparticles are commonly applied in medicine and biology [2][3][4][2,3,4]. Accordingly, the research results have only focused on Au and Ag nanoparticles by modified polyol methods for medical and biological applications. It is obvious that the more complex Pt-based metal nanostructures, typically such as bimetallic and multi-component nanoparticles with alloy or mixing structures, Pt bimetallic shell-core nanostructures, and multi-component nanostructures by modified polyol methods, have not been researched yet, due to the use of much more complex synthesis technologies [14][15][14,15]. The catalytic mechanisms and oxidation of methanol by the crystal planes of Pt nanoparticles were revealed in acid and alkaline electrolyte, changing methanol in to CO2 [15] [15]. Thus, the successful synthesis of Pt-based core-shell nanoparticles with Pt shells of 1–10 nm in new promising properties will open up new and excellent applications that are not available to single-metal nanostructures. Therefore, the as-prepared Pt nanostructures and Pt-based core-shell nanostructures are of particular interest because of their very high practical importance. The main reason is that metal, bimetal, and alloy nanoparticles are potentially used to provide a large extent, and have a wide range in interdisciplinary sciences, typically such as physics, chemistry, electronics, biomedicine, pharmaceuticals, optics-photonics, and catalysis. Typically, Pt-Pd core-shell nanostructures are also nanomaterials that exhibit their outstanding properties. The synergistic properties can be discovered from the Pt shell catalytic property, from the core property, or generated from the co-electrocatalytic properties of both the core and the shell when the Pt-based core and shell nanoparticles are the different catalytic nanomaterials. By changing the shape, structure, size, and composition of the metal core or shell, the electrocatalytic properties of Pt-Pd core-shell nanostructure system can be well controlled. The atom-monolayers shell is an alloy of Pt with another element that also reduces the high cost of the FC system, typically such as Pt3Co, Pt3Ni, Pt3Fe, and Pt3Cu [22]. This means that the price of the Pt catalyst material layer has been reduced by one-third compared with only Pt-based nanostructured catalysts. On that basis, the core-shell nanostructures of the different types of Pt atom-monolayers shells can be studied and developed by physical and chemical methods, such as modified polyol methods. For example, expensive metallic nanoparticles (typically such as Au, and critical elements, such as Pt) are used in order to coat with inexpensive nanoparticles (such as Co, Cu, Ni, Fe etc), leading to the amount of Pt being greatly reduced, but the electrocatalytic properties of the Pt-based catalytic nanoparticles are not less, or even much better. To confirm the catalytic activity of Pt catalyst in the CV cycles, the HER involved in the (111), (100), (111) crystal planes, and other crystal planes of the pure Pt catalyst followed the key reactions of Volmer, Tafel, and Heyrovsky that must be clearly measured as follows.
It is simply emphasized that the electrocatalytic properties of Pt catalyst in acid solution are Pt−Hads→Pt+H++e− (the region is characterized by double–layer charging and discharging), QDL(Charge)↔QDL(Discharge), Pt+H2O→Pt−OH+H++e−, PtOH+H2O→Pt(OH)2+H++e−, Pt−(OH)2→PtO+H2O, 2PtO+4H++4e−→Pt−Pt+2H2O, and Pt+H++e−→Pt−Hads, respectively, [24]that were reviewed [24]. During the catalytic mechanisms and processes, it is confirmed that the Pt catalyst has shown the two peaks of catalytic activity of CH3OH electrooxidation in the CV cycles. Above all, the selectivity, durability, stability, and catalytic activity of multimetal Pt electrocatalysts should need to be certainly verified by a very large number of the CV cycles in order to address the applications of FCs. The high electrochemically active surface area of Pt nanocatalysts, the relationship of high current density vs voltage, the chronoamperometric measurement, or that of current density vs time for a long time must be clearly measured in order to prove in the detail. Similarly, Pt-based multimetal, alloy, and core-shell multimetal nanoparticles need to be intensively confirmed in their high and stable electrocatalytic activity, enough for the applications of FCs.
3. Prospects
The synthetic methods of Pt- or Pd-based bimetal, multimetal, and alloy nanoparticles, as well as hybrid Pt/AB2O4-type ferrite, Pt/ABO3-type perovskite, Pt/oxide, and Pt/ceramic catalysts by modified sol-gel or polyol processes have been reviewed in electrocatalysis [24][24].
- The new Pt-based catalysts in the forms of alloys, metals, multimetal components, oxides, ceramics, and core-shell structures will be prepared by the new, effectively modified polyol and sol-gel process for new FCs, especially for PEMFCs and DMFCs with the use of very low Pt loading.
- The new core-shell-structure nanomaterials will be prepared with metal atomic monolayers from one layer to several layers about 1 nm to 10 nm. The shells can be used as electrocatalysis for chemical reactions.
- The broad application of sol-gel and polyol process for materials and components will be predicted in very promising applications for electronics, photonics, optoelectronics, electronics and telecommunication, engineering and integrated technologies as well as related sciences (Figure 3).

Figure 3. The combined sol-gel and polyol processes for the synthesis of materials and components are predicted in our future [24]. This figure is modified in its original form. [24]. This figure is modified in its original form.
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, J.; Zhang, Z.; Qin, Y.; Wang, Y.-J.; Wang, Y.; Tan, R.; Duan, X.; Tian, T.Z.; Zhang, C.H.; et al. Roadmap: Electrocatalysts for green catalytic processes. J. Phys. Mater. 2021, 4, 022004. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, T.; Kurita, R.; Miyake, M. Shape control of Pt nanoparticles. J. Inorg. Organomet. Polym. Mater. 2000, 10, 145–156. [Google Scholar] [CrossRef]
- Teranishi, T.; Miyake, M. Size control of palladium nanoparticles and their crystal structures. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]
- Ammar, S.; Fiévet, F. Polyol Synthesis: A Versatile Wet-Chemistry Route for the Design and Production of Functional Inorganic Nanoparticles. Nanomaterials 2020, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; Zhao, M.; Yang, T.H.; Gilroy, K.D.; da Silva, A.G.; Camargo, P.H.; Xia, Y. Synthesis of colloidal metal nanocrystals: A comprehensive review on the reductants. Chem. Eur. J. 2018, 24, 16944–16963. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Antolini, E. Platinum-based ternary catalysts for low temperature fuel cells: Part I. Preparation methods and structural characteristics. Appl. Catal. B 2007, 74, 324–336. [Google Scholar] [CrossRef]
- Antolini, E. Platinum-based ternary catalysts for low temperature fuel cells: Part II. Electrochemical properties. Appl. Catal. B 2007, 74, 337–350. [Google Scholar] [CrossRef]
- Viet Long, N.; Minh Thi, C.; Nogami, M.; Ohtaki, M. Pt and Pd Based Catalysts with Novel Alloy and Core-Shell Nanostructures for Practical Applications in Next Fuel Cells: Patents and Highlights. Recent Pat. Mater. Sci. 2012, 5, 175–190. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Thi, C.M.; Van Minh, N.; Cao, Y.; Nogami, M. The development of mixture, alloy, and core-shell nanocatalysts with nanomaterial supports for energy conversion in low-temperature fuel cells. Nano Energy 2013, 2, 636–676. [Google Scholar] [CrossRef]
- Cohen, J.L.; Volpe, D.J.; Abruna, H.D. Electrochemical determination of activation energies for methanol oxidation on polycrystalline platinum in acidic and alkaline electrolytes. Phys. Chem. Chem. Phys. 2007, 9, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and challenges: Review for direct liquid fuel cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C. Prospects for alkaline anion-exchange membranes in low temperature fuel cells. Fuel Cells 2005, 5, 87–200. [Google Scholar] [CrossRef]
- Srivastava, R. Nano-Catalysts for Energy Applications; CRC Press: Boca Raton, FL, USA, 2021; pp. 137–150. [Google Scholar]
- Regalbuto, J. Catalyst Preparation: Science and Engineering; CRC Press: Boca Raton, FL, USA, 2007; pp. 405–448. [Google Scholar]
- Zhang, Y. Bimetallic Nanostructures: Shape-Controlled Synthesis for Catalysis, Plasmonics, and Sensing Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 3–505. [Google Scholar]
- Calvo, F. Nanoalloys: From Fundamentals to Emergent Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 347–376. [Google Scholar]
- Corain, B.; Schmid, G.; Toshim, N. Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–249. [Google Scholar]
- Kumar, S.; Munichandraiah, N.J. Nanoparticles of a Pt3Ni alloy on reduced graphene oxide (RGO) as an oxygen electrode catalyst in a rechargeable Li-O2 battery. Mater. Chem. Front. 2017, 1, 873–878. [Google Scholar] [CrossRef]
-
Hang, N.T.N.; Yang, Y.; Nam, N.Q.T.; Nogami, M.; Phuc, L.H.; Long, N.V. Pt-Based Multimetal Electrocatalysts and Potential Applications: Recent Advancements in the Synthesis of Nanoparticles by Modified Polyol Methods. Crystals 2022, 12, 375. https://doi.org/10.3390/cryst12030375
