Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Vicky Zhou and Version 2 by Vicky Zhou.
Multiple lines of evidence indicated a significant relationship between thyroid carcinomas and other primary extra-thyroidal malignancies (EM), especially breast cancer. For the latter, a prominent association was also found with benign thyroid diseases. Factors other than oncologic treatments may play a role in the initiation and progression of a second primary malignancy. The molecular links between thyroid autoimmunity and breast cancer remain, however, unidentified, and different hypotheses have been proposed.
- thyroid disease
- breast cancer
- etiology
- extra-thyroidal malignancies
1. Introduction
The association of either benign or malignant thyroid diseases (TD) with extra-thyroidal malignancies (EM) has been highlighted in several epidemiological studies, and whether a causal relationship exists between them has been a matter of debate over the last decades. Primarily, such connections have generated much interest around the possibility of identifying common genetic and environmental factors responsible for the etiology and progression of these diseases. In particular, a number of different reports have described the association between thyroid cancers and other primary EM, including breast cancer (BC) [1][2][3][4]. This has led to the hypothesis that the long-term carcinogenic effects of anticancer treatments could be responsible for a second primary cancer. In this area, several researchers evaluated whether the 131I therapy administered to thyroid cancer patients could represent the main cause of a succeeding primary EM. Some of them indicated a 30–42% increased risk of primary EM following 131I exposure, but others did not recognize such correlation. Similarly, studies aimed at clarifying whether anticancer treatments of EM, in particular external beam radiations, may cause subsequent primary thyroid cancers have produced conflicting results. On the other hand, a significant association between benign TD and BC was also shown to occur, which seems to suggest that factors other than oncologic treatment may play a role in the initiation and progression of second malignancies [5][6][7][8]. It has to be mentioned, however, that some biases in the epidemiological studies reporting associations between TD and BC could exist: (i) TD and BC are very common diseases increasing with age in the female population, which make difficult to discern a real link from a chance association; (ii) the majority of studies examining the association of TD with BC are retrospective or cross-sectional and thus more susceptible to biases compared to prospective studies; (iii) both BC and TD are heterogeneous diseases, and only in a minority of reports, the different BC characteristics such as histology and/or molecular subtypes (Figure 1) and/or TD types were examined.
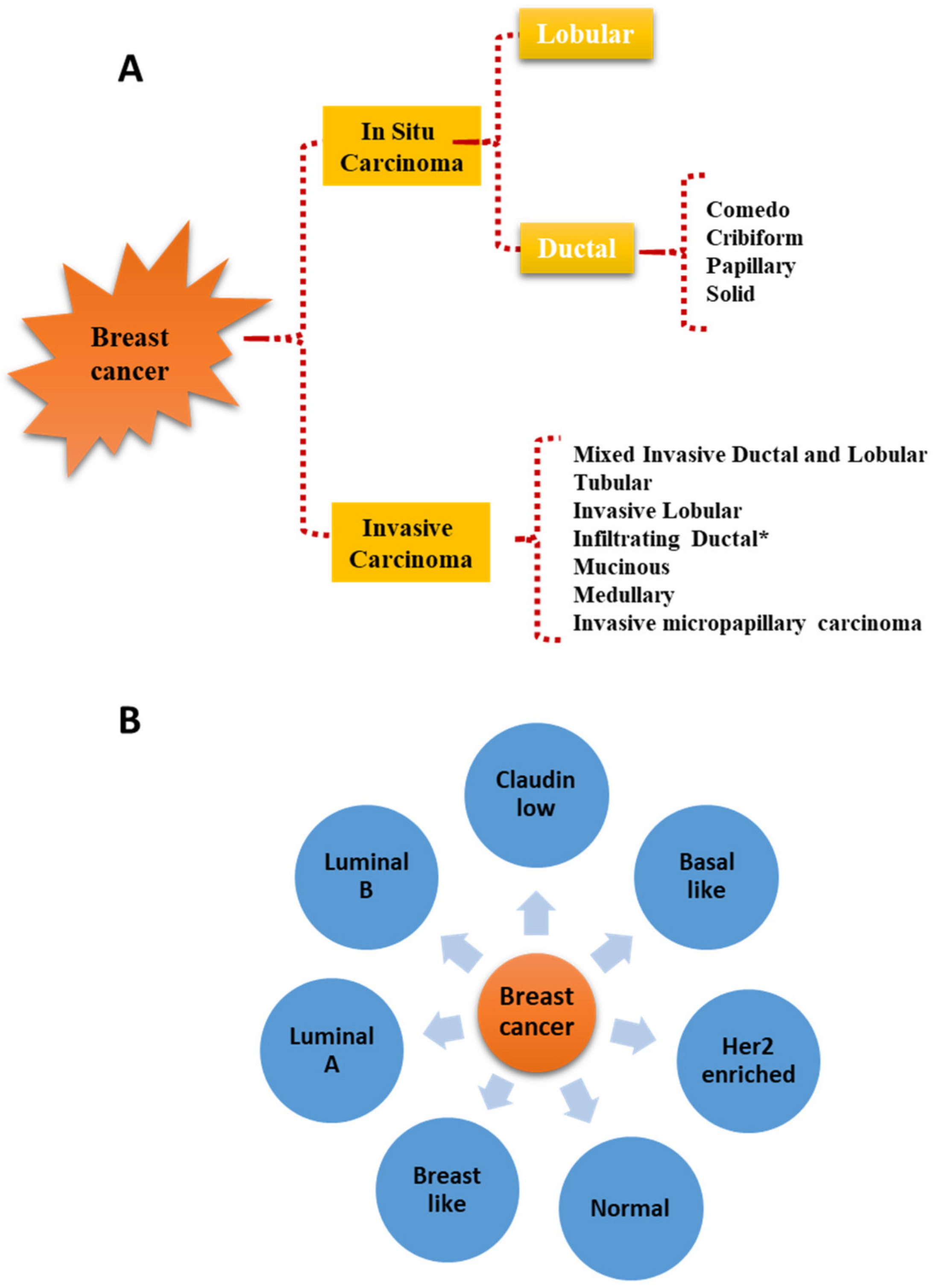 Figure 1. Histological (A) and molecular (B) classification of breast cancer. * Infiltrating ductal carcinomas evaluated on the basis of nuclear morphology, glandular/tubule formation, and mitotic index are further sub-classified in well-differentiated, moderately differentiated, and poorly differentiated carcinomas.
Figure 1. Histological (A) and molecular (B) classification of breast cancer. * Infiltrating ductal carcinomas evaluated on the basis of nuclear morphology, glandular/tubule formation, and mitotic index are further sub-classified in well-differentiated, moderately differentiated, and poorly differentiated carcinomas.2. Thyroid Hormones and Breast Cancer
2.1. Thyroid Hormone Secretion and Mechanisms of Action in Target Tissues
Thyroid hormones are major regulators of growth and development as well as of a number of homeostatic functions in adults, including energy and heat production [9]. Thyroid follicular cells produce two thyroid hormones (THs), 3,5,3′,5′-L-tetraiodothyronine (thyroxine, T4) and 3,5,3′-L-triiodothyronine (T3). Once secreted into the blood, THs are carried by three major transport proteins: thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA or transthyretin), and albumin. The thyroid gland produces about 100 nmol of T4 and 5 nmol of T3 every day to maintain a serum total T4 concentration of about 103 nmol/L and a total T3 concentration of about 1.8 nmol/L [9]. Only 0.04% of total T4 (about 19 pmol/L) and 0.4% of total T3 (about 4.3 pmol/L) circulate in free form and are responsible for the hormonal effects on target tissues [10]. The free THs may act on target cells by two distinct mechanisms: genomic and non-genomic [10][11]. The classical genomic action starts when THs enter the target cells through several plasma membrane transporters, e.g., the monocarboxylate transporters MCT8 and MCT10, the organic anion transporters OATP1 and OATP3, and the L-type amino acid transporter LAT [12]. Once in the cytoplasm, T4 is deiodinated by deiodinases 1 (D1) or 2 (D2) to form T3, which binds TH nuclear receptor (THR) with greater affinity compared to T4 [9]. TH nuclear receptors (THRs) belong to the nuclear receptor superfamily and act as ligand-dependent transcription factors that bind to specific DNA sequences within promoter regions, known as thyroid responsive elements (TRE), and induce or repress the transcription of downstream target genes [13][14]. Two THR proteins have been identified, THRα and THRβ, encoded by the THRA gene, located on chromosome 17, and the THRΒ gene, located on chromosome 3 [15][16][17]. From the THRA gene, three different transcripts are generated, THRα1, THRα2, and THRα3, of which only THRα1 is able to bind T3 [18][19]. Compared to THRα1, THRα2 and THRα3 proteins differ in sequence and in the length of the C-terminal region. The truncated THRα2 and THRα3 receptors can heterodimerize with the full-length receptor and antagonize T3-mediated transcriptional regulation [18][19]. The THRΒ gene provides two receptor isoforms differing in their tissue distribution, THRβ1 and THRβ2, both of which bind T3 [14].
The second mechanism of THs action, by which THs may elicit rapid cellular responses, is initiated at the plasma membrane, where both T4 and T3 may attach to specific regions present in the integrin αvβ3 [10][11][20]. In particular, the latter contains two TH binding sites, termed S1 and S2, of which S1 binds only T3 at physiological serum concentrations and leads to the intracellular activation of the PI3K, while S2 binds T4 and, to a lesser extent, T3, inducing the intracellular activation of extracellular signal-regulated kinases (ERK) 1 and 2 [10][11][21].
2.2. Thyroid Hormones and Breast Development and Cancer
THs have been suggested to play a role, along with other hormones (i.e., prolactin (PRL), estrogen, progesterone, insulin, growth hormone, and adrenal steroids) in normal breast growth and development [22][23]. In particular, high-affinity binding sites for T3 have been identified in the mammary gland and are thought to modulate, upon ligand attachment, ductal branching, alveolar budding, and lobules enlargement [24][25][26][27]. Moreover, in view of their ability to activate PRL plasma membrane receptors and to enhance casein synthesis induced by PRL, THs are considered lactopoietic [22][23][28][29].
The relevance of ERs overexpression during BC progression is well recognized and is such that the most commonly used drugs, i.e., tamoxifen, fulvestrant, and aromatase inhibitors, are aimed at reducing estrogen levels or blocking ER signaling [30][31]. The extensive use of these drugs in the adjuvant therapy of BC is held accountable for the reduced mortality of patients [32][33][34][35][36]. Experimental evidence suggests that TH could support the estrogen-dependent proliferation of BC cells in several ways: (i) TH may increase the expression of estrogen receptors (ERs) [37][38]; (ii) TRE and the ER response element (ERE) share an identical half-site, and THRs have been shown to bind also to ERE [39]; (iii) thyroxine, through the αvβ3 integrin receptor, may activate MAPK signaling and the phosphorylation of the nuclear ERα [40]. This phosphorylation affects ER ability to interact with chromatin, to recruit coregulators, and to modulate gene expression even in the absence of estrogen [40][41][42]. In addition, through integrin receptor signaling, THs were found to favor the proliferation of BC cells lacking ER [43][44]. Other than by their effect on the cell cycle, THs have been shown to prompt BC progression by stimulating aerobic glycolysis (Warburg effect), a hallmark of malignant cells [45][46]; BC cell migration and invasion [45][47]; the expression of Programmed Cell Death Ligand 1 (PD-L1), thus preventing the immune destruction of BC cells [45][48]. These observations are in agreement with a number of recent epidemiological investigations indicating that THs may support BC growth in both pre- and postmenopausal women and with clinical data showing that hypothyroidism may have protective effects by reducing the incidence and progression of BC [6][20][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70]. It is worth considering that T4 maximally stimulates αvβ3 at physiological free-T4 concentrations, while supraphysiological free-T3 concentrations are required to induce cell proliferation via this receptor [42][71][72]. Notably, in a compassionate study comprising patients with far-advanced solid tumors, including BC, Hercbergs and colleagues reported that medically induced euthyroid hypothyroxinemia (pharmacological elimination of T4 and replacement by T3) extended patient’s survival [42][73]. This represents an attractive new therapeutic approach that deserves larger clinical studies to be confirmed.
Nonetheless, it should be taken into account that, while αvβ3 receptors are thought to mediate most of the tumor-promoting effects of THs in BC cells, nuclear THRs appear to play oncosuppressive functions in BC as well as in other solid tumors [74]. The expression of THRs has been documented in BC tissues [75][76]. In particular, Silva and colleagues demonstrated the presence of THRα1 and THRβ1, but not of THRβ2, at both protein and mRNA levels in 70 sporadic BC tissues [75]. However, the loss of the THRΒ gene following truncation or deletion of chromosome 3p, where it is located, or loss of heterozygosity (LOH) and gene rearrangement of the THRA gene have been shown to occur in BC samples [21][74][77]. Somatic mutations of THRs leading to reduced ligand affinity and transcription activity, as well as THRB gene promoter hypermethylation with consequent reduced gene expression, have been also described in BC tissues [74][78][79][80]. The tumor suppressor role of THRΒ has been further validated by Park and colleagues, who overexpressed the THRB gene in the human BC-derived cell line MCF-7, endowed with ER and responsive to estrogen stimulation [81]. In a mouse xenograft model, these MCF-7 cells showed a significantly impaired growth due to reduced proliferation and activation of apoptotic pathways [81].
In conclusion, the imbalance of expression and/or activation between membrane and nuclear TH receptors may have detrimental consequences on BC progression.
3. Autoimmune Thyroid Disease (AITD) and Breast Cancer (BC)
Studies aimed at defining the association between BC and benign TD, in particular AITD, have produced conflicting results causing a long-lasting debate [82][83][84][85][86][87][88][89][90][91][92][93]. In 2002, Sarlis and colleagues performed a meta-analysis of 13 articles published over the previous 50 years including 14,226 women [82]. The authors failed to demonstrate any association between Hashimoto thyroiditis (HT) and BC [82]. Ten years later, Hardefeldt and colleagues accomplished a meta-analysis comprising 28 studies and showed the presence of a higher risk of BC in patients with AITD [7]. In addition, their results testified an increased BC risk associated with the presence of anti-thyroid antibodies and goiter, with Odds Ratios (OR) of 2.92, and 2.26, respectively [7]. The latter data were confirmed in 2020 by Pan and colleagues by means of a meta-analysis on 11 different studies [8]. The authors could establish that patients with BC had higher titers of anti-thyroid peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TgAb) compared to a non-breast disease control group [8]. Similarly, in a very recent meta-analysis involving 21 studies, Chen and colleagues identified TgAb and TPOAb as significantly associated with an increased risk of BC [6]. In ourthe Institute, we of the group, it was analyzed the prevalence of EM in 6386 female patients affected by different TD and weit was found that a number of EM were associated with TD [83][94]. The EM most frequently recorded was BC (OR 3.94), followed by colorectal (OR 2.18), melanoma (OR 6.71), hematological (OR 8.57), uterus (OR 2.52), kidney (OR 3.40), and ovary (OR 2.62) neoplasms. By age-matched analysis, it weas observed that the risk of EM was maximal in the age group 0–44 years (OR 11.28), remaining lower but significantly higher than that observed in the general population in the 45–59 and 60–74-years groups [83]. WeIt was also showedn that when TD patients were dichotomized based on the presence or the absence of TgAb and/or TPOAb, both groups had a higher risk of BC compared to the general population, but the risk was significantly lower in autoantibody-positive patients [83][94]. This finding suggests that amongst TD patients, the presence of thyroid autoantibodies may have a partial protective effect against BC. The latter hypothesis is in agreement with an earlier observation by Smyth and colleagues on TPOAb-positive BC patients, who had a significantly better disease-free and overall survival compared to patients who were TPOAb-negative [84]. In this bacontextkground, the study by Weijl and colleagues reporting the occurrence of hypothyroidism and anti-thyroid antibodies in patients affected by different types of cancer and undergoing immunotherapy with interleukin-2 is of some interest [85]. They found that the preexistence or development of thyroid autoantibodies-related hypothyroidism was associated with a favorable response to immunotherapy [85]. Similar observations were reported by Franzke and colleagues, who observed that autoimmunity caused by IL-2 and IFN-α2 treatment predicted long-term survival in patients affected by metastatic renal cell cancer [86]. To explain the protective role of thyroid autoantibodies, it has been proposed that cell-mediated cytotoxicity elicited by these antibodies against shared antigens may affect the thyroid gland as well as the tumor [87][88]. This hypothesis is consistent with the expression of natrium iodide symporter (NIS) and TPO noticed in breast tissues [87][89]. Despite this evidence, however, further prospective large case studies should be undertaken to definitely prove the protective role of thyroid antibodies in BC cancer progression.
4. Conclusions
The impact of thyroid axis dysfunctions on BC progression has been a matter of debate for more than a century, and still today many controversies exist. The available information strongly suggests that TD may affect BC progression in several ways, through (i) altered plasma levels of deregulated thyrotropin (TSH), and THs or production of specific thyroid autoantibodies; (ii) dysregulation of PRL secretion due to hypothyroidism; (iii) alterations in THs responsiveness of BC cells. Thus, different hormonal and molecular players should be taken into consideration in every single patient, when analyzing the association between TD and BC. This knowledge will likely shed light on the potential pathogenic links between TD and BC, possibly allowing a more personalized clinical management of these patients.
References
- Bhatti, P.; Veiga, L.H.; Ronckers, C.M.; Sigurdson, A.J.; Stovall, M.; Smith, S.A.; Weathers, R.; Leisenring, W.; Mertens, A.C.; Hammond, S.; et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: An update from the childhood cancer survivor study. Radiat. Res. 2010, 174, 741–752.
- Trinh, L.N.; Crawford, A.R.; Hussein, M.H.; Zerfaoui, M.; Toraih, E.A.; Randolph, G.W.; Kandil, E. Deciphering the risk of developing second primary thyroid cancer following a primary malignancy—Who is at the greatest risk? Cancers 2021, 13, 1402.
- Zhang, Y.; Liang, J.; Li, H.; Cong, H.; Lin, Y. Risk of second primary breast cancer after radioactive iodine treatment in thyroid cancer: A systematic review and meta-analysis. Nucl. Med. Commun. 2016, 37, 110–115.
- Mahmood, S.; Vu, K.; Tai, P.; Joseph, K.; Koul, R.; Dubey, A.; Yu, E. Radiation-induced second malignancies. Anticancer Res. 2015, 35, 2431–2434.
- Shu, X.; Ji, J.; Li, X.; Sundquist, J.; Sundquist, K.; Hemminki, K. Cancer risk in patients hospitalised for Graves’ disease: A population-based cohort study in Sweden. Br. J. Cancer 2010, 102, 1397–1399.
- Chen, S.; Wu, F.; Hai, R.; You, Q.; Xie, L.; Shu, L.; Zhou, X. Thyroid disease is associated with increased risk of breast cancer: A systemic review and meta-analysis. Gland. Surg. 2021, 10, 336–346.
- Hardefeldt, P.J.; Eslick, G.D.; Edirimanne, S. Benign thyroid disease is associated with breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2012, 133, 1169–1177.
- Pan, X.F.; Ma, Y.J.; Tang, Y.; Yu, M.M.; Wang, H.; Fan, Y.R. Breast cancer populations may have an increased prevalence of thyroglobulin antibody and thyroid peroxidase antibody: A systematic review and meta-analysis. Breast Cancer 2020, 27, 828–836.
- Greenspan, F.S. The Thyroid Gland. In Basic & Clinical Endocrinology, 7th ed.; Greenspan, F.S., Gardner, D.G., Eds.; Lange Medical Books/McGraw-Hill: New York, NY, USA, 2004; pp. 215–294.
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170.
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043.
- Visser, T.J. Thyroid hormone transporters and resistance. Endocr. Dev. 2013, 24, 1–10.
- Chin, W.W. Nuclear Thyroid Hormone Receptors. In Nuclear Hormone Receptor; Parker, M.G., Ed.; Academic Press: London, UK, 1991; pp. 79–102.
- Ortiga-Carvalho, T.M.; Sidhaye, A.R.; Wondisford, F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014, 10, 582–591.
- Lazar, M.A.; Chin, W.W. Nuclear thyroid hormone receptors. J. Clin. Investig. 1990, 86, 1777–1782.
- Sap, J.; Munoz, A.; Damm, K.; Goldberg, Y.; Ghysdael, J.; Leutz, A.; Beug, H.; Vennström, B. The c-erbA protein is a high-affinity receptor for thyroid hormone. Nature 1986, 324, 635–640.
- Weinberger, C.; Thompson, C.C.; Ong, E.S.; Lebo, R.; Gruol, D.J.; Evans, R.M. The c-erbA gene encodes a thyroid hormone receptor. Nature 1986, 324, 641–646.
- Chassande, O.; Fraichard, A.; Gauthier, K.; Flamant, F.; Legrand, C.; Savatier, P.; Laudet, V.; Samarut, J. Identification of transcripts initiated from an internal promoter in the c-erbA alpha locus that encode inhibitors of retinoic acid receptor-alpha and triiodothyronine receptor activities. Mol. Endocrinol. 1997, 11, 1278–1290.
- Gauthier, K.; Chassande, O.; Plateroti, M.; Roux, J.P.; Legrand, C.; Pain, B.; Rousset, B.; Weiss, R.; Trouillas, J.; Samarut, J. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 1999, 18, 623–631.
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.S.H.; Lai, H.Y.; Whang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid hormone, cancer, and apoptosis. Compr. Physiol. 2016, 6, 1221–1237.
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3,5,3’-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Cell. Physiol. 2009, 296, C980–C991.
- De Sibio, M.T.; De Oliveira, M.; Moretto, F.C.; Olimpio, R.M.; Conde, S.J.; Luvizon, A.C.; Nogueira, C.R. Triiodothyronine and breast cancer. World J. Clin. Oncol. 2014, 5, 503–508.
- Brisken, C.; Ataca, D. Endocrine hormones and local signals during the development of the mouse mammary gland. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 181–195.
- Sellitti, D.F.; Tseng, Y.C.; Latham, K.R. Nuclear thyroid hormone receptors in C3H/HeN mouse mammary glands and spontaneous tumors. Cancer Res. 1983, 43, 1030–1038.
- Hovey, R.C.; Trott, J.F.; Vonderhaar, B.K. Establishing a framework for the functional mammary gland: From endocrinology to morphology. J. Mammary Gland Biol. Neoplasia 2002, 7, 17–38.
- Topper, Y.J.; Freeman, C.S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 1980, 60, 1049–1106.
- Meites, J.; Kragt, C.L. Effects of a pituitary homotransplant and thyroxine on body and mammary growth in immature hypophysectomized rats. Endocrinology 1964, 75, 565–570.
- Bhattacharya, A.; Vonderhaar, B.K. Thyroid hormone regulation of prolactin binding to mouse mammary glands. Biochem. Biophys. Res. Commun. 1979, 88, 1405–1411.
- Borellini, F.; Oka, T. Growth control and differentiation in mammary epithelial cells. Environ. Health Perspect. 1989, 80, 85–99.
- Groner, A.C.; Brown, M. Role of steroid receptor and coregulator mutations in hormone-dependent cancers. J. Clin. Investig. 2017, 127, 1126–1135.
- Tryfonidis, K.; Zardavas, D.; Katzenellenbogen, B.S.; Piccart, M. Endocrine treatment in breast cancer: Cure, resistance and beyond. Cancer Treat. Rev. 2016, 50, 68–81.
- Murphy, C.G.; Dickler, M.N. Endocrine resistance in hormone-responsive breast cancer: Mechanisms and therapeutic strategies. Endocr. Relat. Cancer 2016, 23, R337–R352.
- Castrellon, A.B. Novel strategies to improve the endocrine therapy of breast cancer. Oncol. Rev. 2017, 11, 323.
- Cirocchi, R.; Amabile, M.I.; De Luca, A.; Frusone, F.; Tripodi, D.; Gentile, P.; Tabola, R.; Pironi, D.; Forte, F.; Monti, M.; et al. New classifications of axillary lymph nodes and their anatomical-clinical correlations in breast surgery. World J. Surg. Oncol. 2021, 19, 93.
- Amabile, M.I.; De Luca, A.; Tripodi, D.; D’Alberti, E.; Melcarne, R.; Imbimbo, G.; Picconi, O.; D’Andrea, V.; Vergine, M.; Sorrenti, S.; et al. Effects of inositol hexaphosphate and myo-inositol administration in breast cancer patients during adjuvant chemotherapy. J. Pers. Med. 2021, 11, 756.
- Amabile, M.I.; Frusone, F.; De Luca, A.; Tripodi, D.; Imbimbo, G.; Lai, S.; D’Andrea, V.; Sorrenti, S.; Molfino, A. Locoregional surgery in metastatic breast cancer: Do concomitant metabolic aspects have a role on the management and prognosis in this setting? J. Pers. Med. 2020, 10, 227.
- Ulisse, S.; Tata, J.R. Thyroid hormone and glucocorticoid independently regulate the expression of estrogen receptor in male Xenopus liver cells. Mol. Cell. Endocrinol. 1994, 105, 45–53.
- Alarid, E.T.; Preisler-Mashek, M.T.; Solodin, N.M. Thyroid hormone is an inhibitor of estrogen-induced degradation of estrogen receptor-alpha protein: Estrogen-dependent proteolysis is not essential for receptor transactivation function in the pituitary. Endocrinology 2003, 144, 3469–3476.
- Glass, C.K.; Holoway, J.M. Regulation of gene expression by the thyroid hormone receptor. Biochim. Biophys. Acta 1990, 1032, 157–176.
- Tang, H.Y.; Lin, H.Y.; Zhang, S.; Davis, F.B.; Davis, P.J. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 2004, 145, 3265–3272.
- Hercbergs, A.; Mousa, S.A.; Leinung, M.; Lin, H.Y.; Davis, P.J. Thyroid hormone in the clinic and breast cancer. Horm. Cancer 2018, 9, 139–143.
- Hammes, S.R.; Davis, P.J. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 581–593.
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.Y.; Tang, H.Y.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 2009, 8, 3562–3570.
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front. Endocrinol. 2015, 5, 240.
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones and cancer: A comprehensive review of preclinical and clinical studies. Front. Endocrinol. 2019, 10, 59.
- Suhane, S.; Ramanujan, V.K. Thyroid hormone differentially modulates Warburg phenotype in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 414, 73–78.
- Flamini, M.I.; Uzair, I.; Pennacchio, G.E.; Neira, F.J.; Mondaca, J.M.; Cuello-Carrión, F.D.; Jahn, G.A.; Simoncini, T.; Sanchez, A.M. Thyroid hormone controls breast cancer cell movement via integrin αV/β3/SRC/FAK/PI3-kinases. Horm. Cancer 2017, 8, 16–27.
- Lin, H.Y.; Chin, Y.T.; Nana, A.W.; Shih, Y.J.; Lai, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Davis, P.J. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids 2016, 114, 59–67.
- Angelousi, A.; Diamanti-Kandarakis, E.; Zapanti, E.; Nonni, A.; Ktenas, E.; Mantzou, A.; Kontzoglou, K.; Kouraklis, G. Is there an association between thyroid function abnormalities and breast cancer? Arch. Endocrinol. Metab. 2017, 61, 54–61.
- Khan, S.R.; Chaker, L.; Ruiter, R.; Aerts, J.G.; Hofman, A.; Dehghan, A.; Franco, O.H.; Stricker, B.H.; Peeters, R.P. Thyroid function and cancer risk: The rotterdam study. J. Clin. Endocrinol. Metab. 2016, 101, 5030–5036.
- Journy, N.M.Y.; Bernier, M.O.; Doody, M.M.; Alexander, B.H.; Linet, M.S.; Kitahara, C.M. Hyperthyroidism, hypothyroidism, and cause-specific mortality in a large cohort of women. Thyroid 2017, 27, 1001–1010.
- Cristofanilli, M.; Yamamura, Y.; Kau, S.W.; Bevers, T.; Strom, S.; Patangan, M.; Hsu, L.; Krishnamurthy, S.; Theriault, R.L.; Hortobagyi, G.N. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer 2005, 103, 1122–1128.
- Søgaard, M.; Farkas, D.K.; Ehrenstein, V.; Jørgensen, J.O.; Dekkers, O.M.; Sørensen, H.T. Hypothyroidism and hyperthyroidism and breast cancer risk: A nationwide cohort study. Eur. J. Endocrinol. 2016, 174, 409–414.
- Tosovic, A.; Bondeson, A.G.; Bondeson, L.; Ericsson, U.B.; Malm, J.; Manjer, J. Prospectively measured triiodothyronine levels are positively associated with breast cancer risk in postmenopausal women. Breast Cancer Res. 2010, 12, R33.
- Shi, X.Z.; Jin, X.; Xu, P.; Shen, H.M. Relationship between breast cancer and levels of serum thyroid hormones and antibodies: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 6643–6647.
- Nisman, B.; Allweis, T.M.; Carmon, E.; Kadouri, L.; Maly, B.; Maimon, O.; Meierovich, A.; Peretz, T. Thyroid hormones, silencing mediator for retinoid and thyroid receptors and prognosis in primary breast cancer. Anticancer Res. 2020, 40, 6417–6428.
- Kim, E.Y.; Chang, Y.; Lee, K.H.; Yun, J.S.; Park, Y.L.; Park, C.H.; Ahn, J.; Shin, H.; Ryu, S. Serum concentration of thyroid hormones in abnormal and euthyroid ranges and breast cancer risk: A cohort study. Int. J. Cancer 2019, 145, 3257–3266.
- Ortega-Olvera, C.; Ulloa-Aguirre, A.; Ángeles-Llerenas, A.; Mainero-Ratchelous, F.E.; González-Acevedo, C.E.; Hernández-Blanco, M.L.; Ziv, E.; Avilés-Santa, L.; Pérez-Rodríguez, E.; Torres-Mejía, G. Thyroid hormones and breast cancer association according to menopausal status and body mass index. Breast Cancer Res. 2018, 20, 94.
- Rasool, M.; Naseer, M.I.; Zaigham, K.; Malik, A.; Riaz, N.; Alam, R.; Manan, A.; Sheikh, I.A.; Asif, M. Comparative study of alterations in tri-iodothyronine (T3) and thyroxine (T4) hormone levels in breast and ovarian cancer. Pak. J. Med. Sci. 2014, 30, 1356–1360.
- Tosovic, A.; Bondeson, A.G.; Bondeson, L.; Ericsson, U.B.; Manjer, J. T3 levels in relation to prognostic factors in breast cancer: A population-based prospective cohort study. BMC Cancer 2014, 14, 536.
- Glushakov, R.I.; Proshin, S.N.; Tapil’Skaya, N.I. The incidence of breast tumor during experimental hyperthyroidism. Bull. Exp. Biol. Med. 2013, 156, 245–247.
- Tran, T.V.T.; Maringe, C.; Majano, S.B.; Rachet, B.; Boutron-Rualt, M.C.; Journy, N. Thyroid dysfunction and breast cancer risk among women in the UK biobank cohort. Cancer Med. 2021, 10, 4604–4614.
- Sterle, H.A.; Hildebrandt, X.; Valenzuela Álvarez, M.; Paulazo, M.A.; Gutierrez, L.M.; Klecha, A.J.; Cayrol, F.; Díaz Flaqué, M.C.; Rosemblit, C.; Barreiro Arcos, M.L.; et al. Thyroid status regulates the tumor microenvironment delineating breast cancer fate. Endocr. Relat. Cancer 2021, 28, 403–418.
- Weng, C.H.; Okawa, E.R.; Roberts, M.B.; Park, S.K.; Umbricht, C.B.; Manson, J.E.; Eaton, C.B. Breast cancer risk in postmenopausal women with medical history of thyroid disorder in the women’s health initiative. Thyroid 2020, 30, 519–530.
- Yang, H.; Holowko, N.; Grassmann, F.; Eriksson, M.; Hall, P.; Czene, K. Hyperthyroidism is associated with breast cancer risk and mammographic and genetic risk predictors. BMC Med. 2020, 18, 225.
- Yuan, S.; Kar, S.; Vithayathil, M.; Carter, P.; Mason, A.M.; Burgess, S.; Larsson, S.C. Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: A two-sample Mendelian randomization study. Int. J. Cancer 2020, 147, 1895–1903.
- Tran, T.V.; Kitahara, C.M.; de Vathaire, F.; Boutron-Ruault, M.C.; Journy, N. Thyroid dysfunction and cancer incidence: A systematic review and meta-analysis. Endocr. Relat. Cancer 2020, 27, 245–259.
- Weng, C.H.; Chen, Y.H.; Lin, C.H.; Luo, X.; Lin, T.H. Thyroid disorders and breast cancer risk in Asian population: A nationwide population-based case-control study in Taiwan. BMJ Open 2018, 8, e020194.
- Cordel, E.; Reix, N.; Molière, S.; Mathelin, C. Hyperthyroidism and breast cancer: Is there a link? Gynecol. Obstet. Fertil. Senol. 2018, 46, 403–413.
- Ferreira, E.; da Silva, A.E.; Serakides, R.; Gomes, M.G.; Cassali, G.D. Ehrlich tumor as model to study artificial hyperthyroidism influence on breast cancer. Pathol. Res. Pract. 2007, 203, 39–44.
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871.
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist 2015, 20, 72–76.
- Kim, W.G.; Cheng, S.Y. Thyroid hormone receptors and cancer. Biochim. Biophys. Acta 2013, 1830, 3928–3936.
- Silva, J.M.; Domínguez, G.; González-Sancho, J.M.; García, J.M.; Silva, J.; García-Andrade, C.; Navarro, A.; Muñoz, A.; Bonilla, F. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene 2002, 21, 4307–4316.
- Saraiva, P.P.; Figueiredo, N.B.; Padovani, C.R.; Brentani, M.M.; Nogueira, C.R. Profile of thyroid hormones in breast cancer patients. Braz. J. Med. Biol. Res. 2005, 38, 761–765.
- Chen, L.C.; Matsumura, K.; Deng, G.; Kurisu, W.; Ljung, B.M.; Lerman, M.I.; Waldman, F.M.; Smith, H.S. Deletion of two separate regions on chromosome 3p in breast cancers. Cancer Res. 1994, 54, 3021–3024.
- Futreal, P.A.; Söderkvist, P.; Marks, J.R.; Iglehart, J.D.; Cochran, C.; Barrett, J.C.; Wiseman, R.W. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992, 52, 2624–2627.
- Ling, Y.; Xu, X.; Hao, J.; Ling, X.; Du, X.; Liu, X.; Zhao, X. Aberrant methylation of the THRB gene in tissue and plasma of breast cancer patients. Cancer Genet. Cytogenet. 2010, 196, 140–145.
- Li, Z.; Meng, Z.H.; Chandrasekaran, R.; Kuo, W.L.; Collins, C.C.; Gray, J.W.; Dairkee, S.H. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002, 62, 1939–1943.
- Ling, Y.; Ling, X.; Fan, L.; Wang, Y.; Li, Q. Mutation analysis underlying the downregulation of the thyroid hormone receptor β1 gene in the Chinese breast cancer population. OncoTargets Ther. 2015, 8, 2967–2972.
- Park, J.W.; Zhao, L.; Cheng, S.-Y. Inhibition of estrogen-dependent tumorigenesis by the thyroid hormone receptor b in xenograft models. Am. J. Cancer Res. 2013, 3, 302–311.
- Sarlis, N.J.; Gourgiotis, L.; Pucino, F.; Tolis, G.J. Lack of association between Hashimoto thyroiditis and breast cancer: A quantitative research synthesis. Hormones 2002, 1, 35–41.
- Prinzi, N.; Sorrenti, S.; Baldini, E.; De Vito, C.; Tuccilli, C.; Catania, A.; Coccaro, C.; Bianchini, M.; Nesca, A.; Grani, G.; et al. Association of thyroid diseases with primary extra-thyroidal malignancies in women: Results of a cross-sectional study of 6386 patients. PLoS ONE 2015, 10, e0122958.
- Smyth, P.P.; Shering, S.G.; Kilbane, M.T.; Murray, M.J.; McDermott, E.W.; Smith, D.F.; O’Higgins, N.J. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J. Clin. Endocrinol. Metab. 1998, 83, 2711–2716.
- Weijl, N.I.; Van der Harst, D.; Brand, A.; Kooy, Y.; Van Luxemburg, S.; Schroder, J.; Lentjes, E.; Van Rood, J.J.; Cleton, F.J.; Osanto, S. Hypothyroidism during immunotherapy with interleukin-2 is associated with antithyroid antibodies and response to treatment. J. Clin. Oncol. 1993, 11, 1376–1383.
- Franzke, A.; Peest, D.; Probst-Kepper, M.; Buer, J.; Kirchner, G.I.; Brabant, G.; Kirchner, H.; Ganser, A.; Atzpodien, J. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J. Clin. Oncol. 1999, 17, 529–533.
- Smyth, P.P.A. Autoimmune thyroid disease and breast cancer: A chance association? J. Endocrinol. Investig. 2000, 23, 42–43.
- Rodien, P.; Madec, A.M.; Ruf, J.; Rajas, F.; Bornet, H.; Carayon, P.; Orgiazzi, J. Antibody-dependent cell-mediated cytotoxicity in autoimmune thyroid disease: Relationship to antithyroperoxidase antibodies. J. Clin. Endocrinol. Metab. 1996, 81, 2595–2600.
- Spitzweg, C.; Joba, W.; Eisenmenger, W.; Heufelder, A.E. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J. Clin. Endocrinol. Metab. 1998, 83, 1746–1751.
- Graceffa, G.; Scerrino, G.; Militello, G.; Laise, I.; Randisi, B.; Melfa, G.; Orlando, G.; Mazzola, S.; Cipolla, C.; Cocorullo, G. Breast cancer in previously thyroidectomized patients: Which thyroid disorders are a risk factor? Future Sci. OA 2021, 7, FSO699.
- Bach, L.; Kostev, K.; Schiffmann, L.; Kalder, M. Association between thyroid gland diseases and breast cancer: A case-control study. Breast Cancer Res. Treat. 2020, 182, 207–213.
- Dobrinja, C.; Scomersi, S.; Giudici, F.; Vallon, G.; Lanzaro, A.; Troian, M.; Bonazza, D.; Romano, A.; Zanconati, F.; de Manzini, N.; et al. Association between benign thyroid disease and breast cancer: A single center experience. BMC Endocr. Disord. 2019, 19, 104.
- Rahman, S.; Archana, A.; Jan, A.T.; Dutta, D.; Shankar, A.; Kim, J.; Minakshi, R. Molecular insights into the relationship between autoimmune thyroid diseases and breast cancer: A critical perspective on autoimmunity and ER stress. Front. Immunol. 2019, 10, 344.
- Prinzi, N.; Baldini, E.; Sorrenti, S.; De Vito, C.; Tuccilli, C.; Catania, A.; Carbotta, S.; Mocini, R.; Coccaro, C.; Nesca, A.; et al. Prevalence of breast cancer in thyroid diseases: Results of a cross-sectional study of 3921 patients. Breast Cancer Res. Treat. 2014, 144, 683–688.
More
