Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by ABDULAZIZ BABAIER.
Low-grade serous carcinoma (LGSC) of the ovary is a rare histological subtype of epithelial ovarian carcinoma. It has distinct clinical behavior and a specific molecular profile. Compared with high-grade serous carcinoma, this tumor presents at a younger age, has an indolent course, and is associated with prolonged survival. LGSC can arise de novo or originate following a serous borderline tumor (SBT).
- low-grade serous ovarian carcinoma
- grading system
- pathological features
1. Background
Ovarian cancer remains the most lethal gynecologic malignancy [1]. Epithelial ovarian carcinoma (EOC) is the most frequent histological subtype. Based on histopathology, immunohistochemistry, and molecular analysis, EOCs are divided into five main subtypes: high-grade serous carcinomas (HGSC), endometrioid carcinomas, clear-cell carcinomas, mucinous carcinomas, and low-grade serous carcinomas (LGSC) [2]. HGSC is the most common ovarian epithelial carcinoma, while LGSC is rare. LGSC represents 2–5% of ovarian carcinomas and 5–10% of serous ovarian carcinomas [3][4]. The low prevalence of this disease results in limited data on the disease distribution, specific factors that impact the outcome, and descriptions of patients’ experiences.
Although historically, it was thought that high-grade and low-grade serous tumors existed in a continuum, it has become clear they are two separate entities. They progress through independent pathways and behave differently in their clinical course, and ultimately, in their overall prognosis. LGSC has a distinct clinical behavior and a unique molecular landscape. LGSC is characterized by a younger age at diagnosis, indolent progression, relative chemo-resistance, and long-term survival. The average age at diagnosis is 55.5 years, compared to 62.6 years in HGSC [5]. Other reports even documented a younger median age at diagnosis of 45 years. The disease might affect women as young as 19 years old and as old as 79 years old [6]. The stage distribution of LGSC is similar to HGSC, with 80% of the patients diagnosed at an advanced stage [7]. Despite an equal stage distribution, the survival rate for patients with LGSC is superior (Table 1).
Table 1.
The survival rate for LGSC compared to HGSC
[5]
.
| LGSC | HGSC | |
|---|---|---|
| Overall survival | 99 months | 57 months |
| Stage I | 123 months | 108 months |
| Stage II–IV | 84 months | 52 months |
| 5-year survival | 75% | 40% |
| 10-year survival | 70% | 26% |
| 10-year survival rate stage I | 92% | 76% |
| 10-year survival rate stage II–IV | 55% | 20% |
LGSC: low-grade serous carcinoma; HGSC: high-grade serous carcinoma.
Research identifying the risk factors for developing LGSC has been limited due to the paucity of the disease. Current or previous history of a serous borderline tumor (SBT) of the ovary increases the risk of LGSC. The overall recurrence rate for previously treated SBTs is about 11%, and the absolute rate for malignant transformation of these patients is 2–4% [8]. Longacre et al., in a review of 276 cases, suggested that the potential malignancy risk is up to 6.9% [9]. However, when SBTs with peritoneal implants recur, most are LGSC. Crispen et al. evaluated 53 patients with progressive or recurrent SBTs of the ovary. They observed that 73% of the relapses contained LGSC elements [10]. Although germline BRCA mutations occur in a relatively high proportion of women with HGSC, LGSC does not appear to be part of the hereditary breast-ovarian cancer syndrome. Patients with LGSC are less likely to have a first- or second-degree relative with ovarian cancer [11]. Furthermore, an elevated body mass index (BMI) may increase the risk of LGSC. That could be explained by finding a high number of Müllerian inclusion cysts in the ovary due to the increased level of estrogen and androgens, which could be the origin of low-grade ovarian neoplasm [12].
2. Pathological Aspects
2.1. Grading System for Serous Carcinoma
Traditionally, serous carcinomas of the ovary were categorized according to three three-tier grading systems. These systems were the FIGO (the International Federation of Gynecology and Obstetrics) system, the World Health Organization (WHO) system, and the Shimizu/Silverberg system. The FIGO system evaluated the architectural features of the tumor [13], while the WHO system was based on architectural and cytologic features [14] and the Shimizu/Silverberg system examined three parameters: the glandular architecture, degree of nuclear atypia, and mitotic index [15]. Based on these systems, serous carcinoma would be classified as grade one, two, or three.
In 2004, Malpica et al. defined a novel two-tier system for grading serous ovarian carcinoma into a high grade or low grade [16]. The system is based primarily on the degree of nuclear atypia and uses the mitotic rate as a secondary criterion. In the binary system, tumors with mild to moderate nuclear atypia and a mitotic index of up to 12 mitoses per 10 high-powered fields are classified as low-grade serous cancers. In contrast, tumors with marked nuclear atypia and a mitotic index of >12 mitoses per 10 high-powered fields are classified as high-grade serous cancers. Since its introduction, the system has been validated, allowing its widespread use. The two-tier grading system has proven to have good inter-observer and intra-observer reproducibility [16][17]. Moreover, Bodurka et al. confirmed the superiority of the binary system in predicting clinical outcomes [18]. This user-friendly system has shown a good concordance with other grading systems, with the added advantage of having only two categories [7]. Replacing the historical three-tier systems with the binary system has standardized the diagnosis of LGSC internationally and inspired the medical community to genuinely investigate the difference between HGSC and LGSC in terms of molecular biology and clinical behavior.
2.2. Gross Features
LGSC of the ovary is bilateral in >50% of the cases. The size might range from 1 cm to more than 20 cm [6][19]. It is usually multicystic, involving the ovarian surface and parenchyma with papillary excrescences. The contents are serosanguinous or watery, straw-colored, and less frequently mucinous [6].
2.3. Microscopic Features
Accurate pathological evaluation is crucial to managing LGSC properly. It typically shows uniform cells with frank destructive invasion associated with mild to moderate atypia and a low mitotic index.
LGSC is characterized by a monotonous population of cuboidal, low columnar, and sometimes flattened cells with an amphophilic or lightly eosinophilic cytoplasm. The degree of atypia is mild to moderate, with evenly distributed chromatin; occasional cells with larger nuclei can be seen. As mentioned above, the number of mitoses is no more than 12 mitoses per 10 high-power fields. Destructive invasion is recognized by neoplastic cells in the tumor/ovarian stroma in an area that measures ≥3.0 mm in linear dimension or has desmoplasia. The invasive component may grow in various architectural patterns such as micropapillary, cribriform, elongated papillae, glandular, medium-sized papillae, nests, macro-papillae, cell clusters, and as single cells (Figure 1). Commonly, there is a mix of architectural patterns. Psammoma bodies are common in LGSC and may be numerous. Occasionally, extracellular and intracellular mucin can be observed. Necrosis or multinucleated tumor giant cells are not commonly seen [6][7][19][20]. Sporadically, LGSC is associated with high-grade serous carcinoma at presentation or recurrences [21].
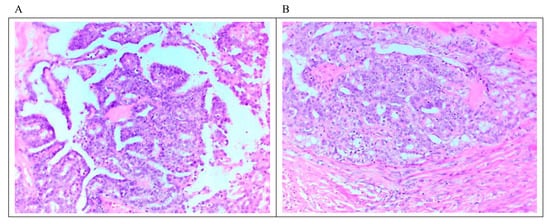

Figure 1. Microscopic feature of LGSC. (A) Micropapillary architecture outlined by cuboidal cells with mild to moderate atypia associated with low mitosis. (B) Focus on stromal invasion by the tumor with a cribriform architectural pattern. Scale bar 10 μm (20 × magnification).
LGSC may primarily originate from the peritoneum rather than the ovary. Primary peritoneal carcinoma (PPC) is diagnosed based on the gynecology oncology group (GOG) criteria. The ovarian component must be nonexistent, confined to the surface with no cortical invasion, or involving the ovarian surface and underlying cortical stroma without any focus on the stroma, measuring >5 mm in depth and width [22]. Once the criteria for LGSC and PPC are met, the diagnosis of LGSC of the peritoneum is made.
Psammocarcinoma is a rare variant of serous neoplasm arising from the ovary or peritoneum that is separate from LGSC. It is described as a serous neoplasm exhibiting the following pathological features: (a) destructive invasion, (b) no more than moderate atypia, (c) no areas of solid epithelial proliferation except for occasional nests no more than 15 cells in diameter, and (d) at least 75% of papillae or nests associated with or entirely replaced by psammoma bodies. Psammocarcinoma has limited malignant potential and a favorable prognosis [23][24].
2.4. Immunohistochemical (IHC) Profile
IHC analysis has become an integral part of the pathological assessment. Therefore, understanding the staining pattern of LGSC should facilitate reaching a definite diagnosis. LGSC is usually stained positively for Wilson tumor-1 protein (WT-1), a marker used to confirm serous differentiation, and PAX-8, an indicator used to confirm a Müllerian origin [25]. In addition, most cases express estrogen receptors (ER), and some express progesterone receptors (PR) and E- cadherin. PAX-2 is overexpressed in 67% of the SBT and 50% of LGSC but never expressed in HGSC [26].
Her-2/neu expression is detected in about 28% of the patients and c-kit is positive in 4.5% of the cases [27]. Furthermore, P16 expression is mainly patchy or focal but sometimes can be diffuse or strong [28]. P53 expression is mostly wild-type but it might be overexpressed in 18% of the cases [25][29]. Rare cases of LGSC with the BRAF mutation are positive for VE1 (BRAF V600E) protein expression [30]. The Ki-67 proliferative index is typically positive in less than 10% of the tumor cells, while a higher index can also be seen [25][28]. Table 32 summarizes the typical LGSC IHC profile in relation to HGSC.
Table 32.
Typical IHC profile of LGSC compared to HGSC.
| Biomarker | LGSC | HGSC |
|---|---|---|
| WT-1 | Positive | Positive |
| PAX-8 | Positive | Positive |
| ER | Mostly Positive | Mostly Positive |
| PR | Possibly Positive (~50%) | Possibly Positive (~30%) |
| MIB1 | Mainly negative | Possibly positive (~50%) |
| E-cadherin | Possibly Positive | Mainly negative |
| PAX-2 | Positive (~50%) | Negative |
| Her-2/neu | Possibly positive (~30%) | Possibly positive (~20%) |
| P16 | Mainly patchy or Negative | Diffusely positive |
| P53 | Mainly patchy or Negative | Diffusely positive (~90%) |
| Ki-67 | Low | High |
LGSC: low-grade serous carcinoma; HGSC: high-grade serous carcinoma; WT-1; Wilson tumor-1 protein; ER: estrogen receptor; PR: progesterone receptor.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. Cancer J. Clin. 2021, 71, 7–33.
- WHO Classification of Tumours Editorial Board. Female Genital Tumours: WHO Classification of Tumours, 5th ed.; IARC: Lyon, France, 2020; Volume 4.
- Matsuo, K.; Machida, H.; Grubbs, B.H.; Sood, A.K.; Gershenson, D.M. Trends of low-grade serous ovarian carcinoma in the United States. J. Gynecol. Oncol. 2018, 29, e15.
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The Histologic Type and Stage Distribution of Ovarian Carcinomas of Surface Epithelial Origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44.
- Plaxe, S.C. Epidemiology of low-grade serous ovarian cancer. Am. J. Obstet. Gynecol. 2008, 198, 459.e1–459.e9.
- Okoye, E.; Euscher, E.D.; Malpica, A. Ovarian low-grade serous carcinoma. A clinicopathologic study of 33 cases with primary surgery performed at a single institution. Am. J. Surg. Pathol. 2016, 40, 627–635.
- Malpica, A.; Deavers, M.T.; Lu, K.; Bodurka, D.; Atkinson, E.N.; Gershenson, D.M.; Silva, E.G. Grading Ovarian Serous Carcinoma Using a Two-Tier System. Am. J. Surg. Pathol. 2004, 28, 496–504.
- Du Bois, A.; Ewald-Riegler, N.; de Gregorio, N.; Reuss, A.; Mahner, S.; Fotopoulou, C.; Kommoss, F.; Schmalfeldt, B.; Hilpert, F.; Fehm, T.; et al. Borderline tumours of the ovary: A cohort study of the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Study Group. Eur. J. Cancer 2013, 49, 8.
- Longacre, T.A.; McKenney, J.K.; Tazelaar, H.D.; Kempson, R.L.; Hendrickson, M.R. Ovarian Serous Tumors of Low Malignant Potential (Borderline Tumors). Am. J. Surg. Pathol. 2005, 29, 707–723.
- Crispens, M.; Bodurka, D.M.; Lu, K.; Silva, E.G.; Gershenson, D.M. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstet. Gynecol. 2002, 99, 3–10.
- Vineyard, M.A.; Daniels, M.S.; Urbauer, D.L.; Deavers, M.T.; Sun, C.C.; Boerwinkle, E.; Bodurka, D.C.; Gershenson, D.M.; Crawford, J.; Lu, K.H. Is low-grade serous ovarian cancer part of the tumor spectrum of Hereditary Breast and Ovarian Cancer? Gynecol. Oncol. 2011, 120, 229–232.
- Folkins, A.K.; Saleemuddin, A.; Garrett, L.A.; Garber, J.E.; Muto, M.G.; Tworoger, S.S.; Crum, C.P. Epidemiologic correlates of ovarian cortical inclusion cysts (CICs) support a dual precursor pathway to pelvic epithelial cancer. Gynecol. Oncol. 2009, 115, 108–111.
- FIGO Committe on Gynecologic Oncology. FIGO staging classifications and clinical practice gudelines in the management of gynecologic cancers. Int. J. Gynecol. Obstet. 2000, 70, 2.
- Scully, R.E. Histological typing of ovarian tumors. In World Health Organization International Histological Classification of Tumors, 2nd ed.; Springer: New York, NY, USA, 1999.
- Shimizu, Y.; Kamoi, S.; Amada, S.; Akiyama, F.; Silverberg, S.G. Toward the development of a universal grading system for ovarian epithelial carcinoma. Cancer 1998, 82, 5.
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747.
- Seidman, J.D.; Horkayne-Szakaly, I.; Cosin, J.A.; Ryu, H.S.; Haiba, M.; Boice, C.R.; Yemelyanova, A.V. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol. Oncol. 2006, 103, 703–708.
- Bodurka, D.C.; Deavers, M.T.; Tian, C.; Sun, C.C.; Malpica, A.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Birrer, M.J.; Ozols, R.; et al. Reclassification of serous ovarian carcinoma by a 2-tier system. Cancer 2012, 118, 3087–3094.
- Vang, R.; Shih, I.-M.; Kurman, R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282.
- Ahn, G.; Folkins, A.K.; McKenney, J.K.; Longacre, T.A. Low-grade Serous Carcinoma of the Ovary: Clinicopathologic Analysis of 52 Invasive Cases and Identification of a Possible Noninvasive Intermediate Lesion. Am. J. Surg. Pathol. 2016, 40, 1165–1176.
- Dehari, R.; Kurman, R.J.; Logani, S.; Shih, I.-M. The Development of High-grade Serous Carcinoma from Atypical Proliferative (Borderline) Serous Tumors and Low-grade Micropapillary Serous Carcinoma. Am. J. Surg. Pathol. 2007, 31, 1007–1012.
- Bloss, J.D.; Liao, S.-Y.; Buller, R.E.; Manetta, A.; Berman, M.L.; McMeekin, S.; Bloss, L.P.; DiSaia, P.J. Extraovarian Peritoneal Serous Papillary Carcinoma: A Case-Control Retrospective Comparison to Papillary Adenocarcinoma of the Ovary. Gynecol. Oncol. 1993, 50, 347–351.
- Rettenmaier, M.A.; Goldstein, B.H.; Epstein, H.D.; Brown, J.V.; Micha, J.P. Serous psammocarcinoma of the ovary: An unusual finding. Gynecol. Oncol. 2005, 99, 510–511.
- Bilgin, T.; Özuysal, S.; Cankiliç, H. Primary psammocarcinoma of the peritoneum. Int. J. Gynecol. Cancer 2006, 16, 129–131.
- O’Neill, C.J.; Deavers, M.T.; Malpica, A.; Foster, H.; McCluggage, W.G. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: Significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am. J. Surg. Pathol. 2005, 29, 1034–1041.
- Tung, C.S.; Mok, S.C.; Tsang, Y.T.M.; Zu, Z.; Song, H.; Liu, J.; Deavers, M.T.; Malpica, A.; Wolf, J.K.; Lu, K.H.; et al. PAX2 expression in low malignant potential ovarian tumors and low-grade ovarian serous carcinomas. Mod. Pathol. 2009, 22, 1243–1250.
- Wong, K.-K.; Lu, K.H.; Malpica, A.; Bodurka, D.C.; Shvartsman, H.S.; Schmandt, R.E.; Thornton, A.D.; Deavers, M.T.; Silva, E.G.; Gershenson, D.M. Significantly Greater Expression of ER, PR, and ECAD in Advanced-Stage Low-Grade Ovarian Serous Carcinoma as Revealed by Immunohistochemical Analysis. Int. J. Gynecol. Pathol. 2007, 26, 404–409.
- O’Neill, C.J.; McBride, H.A.; Connolly, L.E.; Deavers, M.T.; Malpica, A.; McCluggage, W.G. High-grade ovarian serous carcinoma exhibits significantly higher p16 expression than low-grade serous carcinoma and serous borderline tumour. Histopathology 2007, 50, 773–779.
- Altman, A.D.; Nelson, G.S.; Ghatage, P.; McIntyre, J.B.; Capper, D.; Chu, P.; Nation, J.G.; Karnezis, A.N.; Han, G.; Kalloger, S.E.; et al. The diagnostic utility of TP53 and CDKN2A to distinguish ovarian high-grade serous carcinoma from low-grade serous ovarian tumors. Mod. Pathol. 2013, 26, 1255–1263.
- Turashvili, G.; Grisham, R.N.; Chiang, S.; DeLair, D.F.; Park, K.J.; Soslow, R.A.; Murali, R. BRAF V 600E mutations and immunohistochemical expression of VE1 protein in low-grade serous neoplasms of the ovary. Histopathology 2018, 73, 438–443.
More
