Energetic materials (EMs) are widely used in military and civilian applications, such as weaponry, aerospace explorations and fireworks. However, both the power and safety of energetic materials are the most concern in the field of energetic materials application, but there is an essential contradiction between them: the highly energetic materials are often not safe, and, at present, the rareness of pure low-sensitive and highly energetic explosive has been found. Due to the stringent requirements for both low-sensitivity and high-power simultaneously, the cocrystallization of explosive has attracted great interest since it can alleviate, to a certain extent, the power-safety contradiction. The new energetic cocrystals can potentially exhibit decreased sensitivity, higher performance through increased packing efficiency or oxygen balance, or improve aging due to altered bonds to specific groups on constituent molecules.
- molecular dynamic simulation
- CL-20
- cocrystal energetic materials
- preparation
- characterization
1. Introduction
Energetic materials (EMs) are widely used in military and civilian applications, such as weaponry, aerospace explorations and fireworks. However, both the power and safety of energetic materials are the most concern in the field of energetic materials application, but there is an essential contradiction between them: the highly energetic materials are often not safe, and, at present, the rareness of pure low-sensitive and highly energetic explosive has been found [1][2][3][1,2,3]. For a long time, in order to obtain EMs with lower sensitivity, the modifications of existing explosives have often focused mainly on recrystallizing with solution and coating with polymer [4][5][6][4,5,6]. However, these traditional methods cannot markedly reduce the sensitivities of existing explosives by only modifying morphology or diluting power, due to the unchanging inherent structures of explosive molecules [7][8][7,8]. Due to the stringent requirements for both low-sensitivity and high-power simultaneously, the cocrystallization of explosive, a technique by which a multi-component crystal of several neutral explosive molecules forms in a defined ratio through non-covalent interactions (e.g., H-bond, electrostatic interaction, etc.) [9][10][9,10], has attracted great interest since it can alleviate, to a certain extent, the power-safety contradiction [11][12][11,12]. The new energetic cocrystals can potentially exhibit decreased sensitivity, higher performance through increased packing efficiency or oxygen balance, or improve aging due to altered bonds to specific groups on constituent molecules [13][14][15][13,14,15]. Recently a lot of cocrystal explosives have been synthesized and characterized [16][17][18][19][20][16,17,18,19,20]. An evaluation of the power and safety of energetic cocrystals has been carried out, and cocrystallization is becoming increasingly hot in the field of energetic materials [21]. It was found that the stability (sensitivity) and detonation performance (energy, detonation velocity, and detonation pressure, etc.) of cocrystal explosive could be influenced by the molar ratio of molecular combination. Generally, when a cocrystal has too much content of high-energy explosives, the packing density and detonation performance will be increased, with possible increase in explosive sensitivity. On the contrary, the sensitivity will be decreased in a cocrystal explosive with a high content of low-energetic or non-energetic explosives. The searches for stable insensitive and highly-energetic explosive is the primary goal in the field of energetic material chemistry. Therefore, the molar ratios of two or more kinds of explosive components should be controlled in a reasonable scope, and it is very necessary to clarify the influence of the ratio of molecular combination on the stability and detonation performance of cocrystal explosives, such as packing density, oxygen balance, detonation velocity, and detonation pressure, etc. [22].
The compound 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) is currently known to be one of the most powerful explosives [23], and it is also a superior alternative to HMX for applications in low signature rocket propellants [24][25][26] [24,25,26] and gun propellants [27]. However, the disadvantage of CL-20 is its increased sensitivity to mechanical stimuli and cocrystallization of CL-20 with other compounds, which may provide a way to decrease its sensitivity [28]. Moreover, the relationship of the reaction kinetic process with temperatures and densities for pyrolysis of CL-20/TNT cocrystal was investigated using a reactive force field (ReaxFF) molecular dynamics simulation. The evolution distribution of potential energy and total species, decay kinetics and kinetic parameters for thermal decomposition reaction of CL-20 and TNT were analyzed. It was shown that the breaking of −NO2 bond from CL-20 molecules is the initial reaction pathway for the thermal decomposition of the cocrystal. With increasing the cocrystal density, the reaction energy barrier of CL-20 and TNT molecule decomposition increases correspondingly. The decomposition process of TNT has an inhibition action on the decomposition of CL-20 [29]. Meanwhile, the combustion behavior, flame structure, and thermal decomposition of bi-molecular crystals of CL-20 with glycerol triacetate (GTA), tris [1][2][5][1,2,5]oxadiazolo [3,4-b:3′,4′-d:3′’,4′’-f]azepine-7-amine (ATFAz), 4,4′’-dinitro-ter-furazan (BNTF), oxepino [2,3-c:4,5-c’:6,7-c’’] trisfurazan (OTF), oxepino [2,3-c:4,5-c’:6,7-c’’] trisfurazan-1-oxide (OTFO) were studied [30]. It was found that the introduction of volatile and thermally stable compounds into the composition with CL-20 decreased the thermal stability of CL-20. The combustion mechanism of the CL-20 cocrystal depends both on the burning rate of the second component and its volatility. While there are few overview papers on CL-20-based cocrystal’s simulation, preparation and characterization. Thus, in this paper, the achievements in the intermolecular interaction of five types of CL-20-based cocrystals (CL-20/TNT, CL-20/HMX, CL-20/FOX-7, CL-20/TKX-50 and CL-20/DNB) by using molecular dynamic simulation are reviewed. The preparation and performance of CL-20-based cocrystals are emphatically analyzed, and the existing problems and challenges in the future work are reviewed. The aim of this work is mainly to explore the nature of the formation of the different CL-20-based cocrystal morphology and clarify the influence of the ratio of molecular combination on the stability and detonation performance of CL-20-based cocrystal explosives.
2. Energetic Performance
In order to study the thermal decomposition properties of CL-20/TNT cocryatal explosives, the samples of CL-20/TNT cocryatals were tested by DSC (TA Instruments, New Castle, DE, USA) at the heating rate of 10 °C·min−1, the curve shown in Figure 14. As is shown there are three stages of the thermal decomposition process of CL-20/TNT cocrystal, the maximum endothermic peak temperature is 143.5 °C, which is higher than that of the melting point of TNT (81 °C [31][37]). With the increase of temperature, the hydrogen bond between the cocrystal molecules breaks and the molecular structure is destroyed [32][38]. There are two processes of exothermic decomposition at 222.6 °C and 250.1 °C, respectively. Compared with the maximum exothermic peak value of CL-20 (321.5 °C [33]) and TNT (245 °C [34][39]), the exothermic peak of CL-20/TNT cocrystal shifts, indicating that the cocrystal changes the thermal decomposition characteristics of the raw materials and endows the cocrystal with unique thermal decomposition behavior.
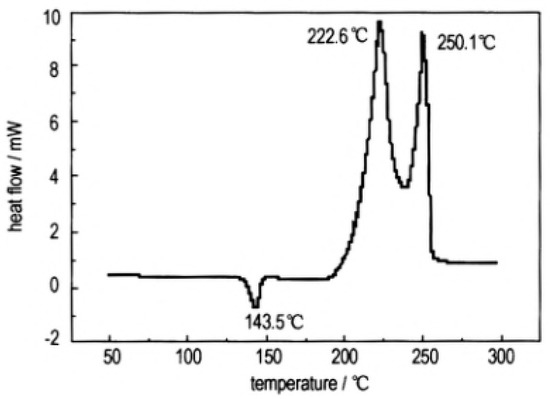
Figure 14. DSC curve of the CL-20/TNT cocrystal explosive [8], with permission from Han Neng Cai Liao, 2012.
The impact sensitivity value of CL-20/TNT cocrystal is H50 = 28 cm. Compared with CL-20 (H50 = 15 cm), the impact sensitivity of CL-20 is significantly reduced by 87%. Low-sensitivity TNT and high-sensitivity CL-20 combine to form a cocrystal at the molecular scale through eutectic technology, which changes the internal composition and crystal structure of explosives compared with traditional methods. In addition, due to the intermolecular hydrogen bond, on the one hand, it increases the stability of the molecular system of the cocrystal, on the other hand, it improves the anti-vibration of the cocrystal molecule to the mechanical external force, so the desensitization effect is obvious. Through the eutectic technology, it can effectively realize the desensitization of high sensitive explosive and improve its safety performance.
The crystal morphology, particle size and sensitivity of prepared CL-20/HMX cocrystal were characterized [6]. The thermal decomposition process of cocrystal explosives had only one exothermic decomposition stage with peak temperatures of 248.3 °C. The enthalpy for the exothermic decomposition of the cocrystal (2912.1 J·g−1) was remarkably higher than that of the physical mixture of CL-20 and HMX (1327.3 J·g−1) (Figure 215). The friction sensitivity of CL-20/HMX cocrystal explosive was 84%, which was decreased by 16% compared with original CL-20, and the characteristic height of the cocrystal was increased by 28.6 cm and 11.5 cm compared with original CL-20 and HMX, respectively (Table 18). The compatibility of CL-20/HMX cocrystal with components of solid propellant shown that the prepared CL-20/HMX cocrystal was compatible with NG/BTTN, AP and Al powder, while incompatible with HGAP, N-100.
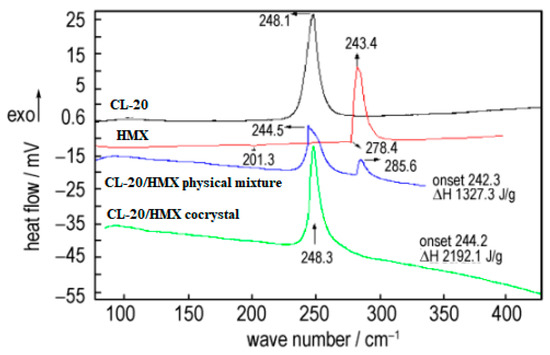
Figure 215. DSC curves of different samples [6], with permission from Han Neng Cai Liao, 2020.
Table 18. The mechanical sensitivities of different samples.
| Samples | F | /% | H50 | /cm | P | /MPa | D | /m·s | −1 |
|---|---|---|---|---|---|---|---|---|---|
| CL-20 | 100 [6][7] | 100 [6,7] | 19.3, 13.1 [20]; 13.1 [7]; 15 [8][9][35] | 19.3, 13.1 [20]; 13.1 [7]; 15 [8,9,34] | 44.9 [35]; 43 [8] | 44.9 [34]; 43 [8] | 9385 [35]; 9500 [8] | 9385 [34]; 9500 [8] | |
| HMX | 28 [6]; 84 [7] | 36.4 [6]; 19.6 [7]; 27.2 [9]; 13.9 [34] | 39.6 [35] | 39.6 [34] | 9048 [35] | 9048 [34] | |||
| Ball milling of CL-20 | 72 [7] | 42.3 [7] | |||||||
| Ball milling of HMX | 60 [7] | 47.8 [7] | |||||||
| CL-20/HMX mixture | 96 (mole ratio is 2:1) [7] | 15.4 (mole ratio is 2:1) [7], 20.1 [9] | |||||||
| Ball milling of CL-20/HMX mixture (mole ratio is 2:1) | 68 [7] | 43.5 [7] | |||||||
| Micro/nano CL-20/HMX cocrystal | 60 [7] | 47.6 [7], 32.6 [9] | |||||||
| Ultrafine CL-20/HMX cocrystal | 84 [6] | 47.9 [6] | |||||||
| TNT | 102 [8], 157.2 [20] | 21 [8] | 6900 [8] | ||||||
| CL-20/TNT cocrystal | 28 [8], 49.3 [20] | 35 [8] | 8600 [8] | ||||||
| CL-20/TNT mixture | 18.8 [20] | ||||||||
| TKX-50 | 55.4 [35] | 55.4 [34] | 41.0 [35] | 41.0 [34] | 8524 [35] | 8524 [34] | |||
| CL-20/TKX-50 mixture | 26.0 [35] | 26.0 [34] | |||||||
| CL-20/TKX-50 cocrystal | 34.0 [35] | 34.0 [34] | 43.8 [35] | 43.8 [34] | 9264 [35] | 9264 [34] |
H50 is characteristic drop height, cm; F is friction sensitivity, %; D is detonation velocity, m·s−1; P is detonation pressure, MPa. The hammer weight: (2.500 ± 0.002) kg, sample weight: (35 ± 1) mg for impact sensitivity in [7][20][7,20], the hammer weight: (1.5 ± 0.01) kg, sample weight: (20 ± 1) mg, pressure: (2.45 ± 0.07) MPa, swing angle: (80 ± 1)0 for friction sensitivity in [7]; the hammer weight: 2 kg, sample weight: 30 mg for impact sensitivity, for friction sensitivity in [6] [6]; the hammer weight: 2 kg, sample weight: (30 ± 1) mg in [8]; the hammer weight: 5 kg, sample weight: (35 ± 1) mg in [9][35][9,34].
At the same time, the DSC curves of CL-20/HMX cocrystal were prepared by means of mechanical ball milling, and compared with the CL-20/HMX mixture [7]. The mechanical sensitivity of the micro/nano CL-20/HMX energetic cocrystal materials was reduced obviously compared to that of raw HMX, while the energy output property was equivalent to that of raw CL-20.
In order to compare the effect of the prepared CL-20/TKX-50 cocrystal on the thermal decomposition of each component, the DSC curves of CL-20, TKX-50, CL-20/TKX-50 mixture and CL-20/TKX-50 cocrystal were investigated [35] [34] (Figure 316). The exothermic decomposition peaks of CL-20 and TKX-50 were 240.2 and 234.8 °C, respectively, and there were two exothermic decomposition peaks in the decomposition process of the CL-20/TKX-50 mixture, indicating that the decomposition process of the CL-20/TKX-50 mixture is obviously a simple superposition of CL-20 and TKX-50. The first decomposition peak appears at 229.8 °C, corresponding to the exothermic decomposition of part of TKX-50; the second peak appears at 242.5 °C, corresponding to the exothermic decomposition of CL-20. For the CL-20/TKX-50 cocrystal decomposition, with the increase of temperature, the first exothermic decomposition peak of CL-20/TKX-50 cocrystal appears at 171.6 °C, which can be attributed to the destruction of hydrogen in the cocrystal structure and the decomposition of a small amount of TKX-50. Subsequently, a large number of cocrystal products begin to decompose, and the second exothermic decomposition peak appeared at 222.8 °C, indicating the formation of hydrogen bond and the existence of a new structure between CL-20/TKX-50 cocrystal molecules.
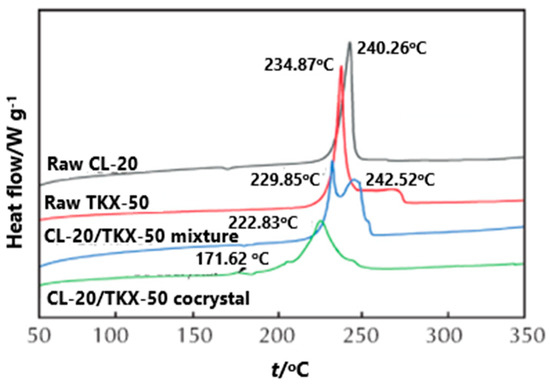
Figure 316. DSC curves of CL-20, TKX-50, CL-20/TKX-50 mixture and CL-20/TKX-50 cocrystal [35][34], with permission from Huo Zha Yao Xue Bao, 2020.
In addition, the DSC curves of CL-20, DNB, CL-20/DNB cocrystal were shown in Figure 417. There are three (one is endothermic melting and two exothermic decompositions) thermal decomposition stages. The melting temperature of cocrystal is 136.4 °C, 44.7 °C higher than that of raw material DNB (91.7 °C) in the melting stage, indicating that the cocrystal decomposes into liquid DNB at this point. There are two exothermic peaks at 216.9 °C and 242.9 °C in the exothermic decomposition stage, which was attributed to the exothermic decomposition of two single components after cocrystal decomposition. Moreover, the crystal density of CL-20/DNB is higher than that of CL-20/TNT, and the sensitivity of DNB is lower than that of TNT. Thus, the sensitivity of CL-20/DNB is lower than that of CL-20/TNT. In addition, the cost of the DNB is significantly lower than that of TNT. Therefore, the cocrystal may become an excellent explosive with high-energy, insensitive and low-cost prospective ingredients in explosives and propellants.
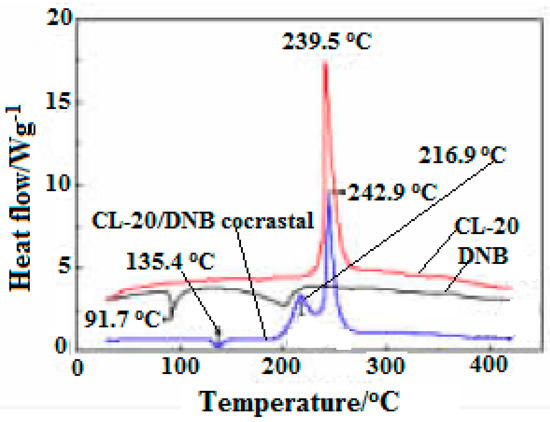
Figure 417. DSC curves of CL-20, DNB and CL-20/DNB cocryatal [5], with permission from Chinese Journal of Energetic Materials, 2013.
The impact sensitivity, the calculated detonation of the CL-20, TKX-50, CL-20/TKX-50 mixture and CL-20/TKX-50 cocrystal are shown in Table 8. The characteristic drop height of CL-20/TKX-50 cocrystal is lower than that of TKX-50, but significantly higher than that of CL-20, β-HMX and CL-20/TKX-50 mixture, indicating that the impact sensitivity of CL-20/TKX-50 cocrystal is much lower. The detonation velocity and detonation pressure of CL-20/TKX-50 cocrystal are slightly lower than that of CL-20, but the detonation performance is obviously improved compared with β-HMX, indicating CL-20/TKX-50 cocrystal has good detonation performance.
3. Existing Problems and Challenges
As we all know, it is of great significance for the preparation of energetic materials with high-energy and low-sensitivity to use eutectic technology to modify the single high-energy explosives. While the development of an energetic cocrystal is still in the exploratory stage, the main challenges for cocrystal at present are as follows: (1) the optimal preparation method of energetic cocrystal still needs to be simplified and simulated. The solvent evaporation method is mainly used to explore the preparation conditions of cocrystal by experience at present and many experiments with different stoichiometric ratios cannot meet the engineering requirements. (2) The effective cocrystal formation principle and formation mechanism of energetic compounds under the guidance of thermodynamics are not fully revealed, which greatly hinders the controllable construction of energetic cocrystal compounds [36][40]. The main challenges for us in the future are possibly: (1) the design and formation mechanism of energetic cocrystal can be draw lessons from the other research fields, the cocrystal is formed by self-assembly of hydrogen bonding and π–π stacking interactions; (2) on the basis of the existing mature eutectic technology, a safe, efficient and practical synthesis method suitable for energetic cocrystal should be improved, promoting the theoretical research of cocrystal formation, and simulate and design the formation of energetic cocrystal from the theoretical level [37][41].
