Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Afaf HAMAME and Version 2 by Rita Xu.
Colistin (polymyxin E) has been used as a growth promoter in food-production animals, but it is also used in pets for the prevention and treatment of bacterial infections.
- colistin resistance
- mcr genes
- dogs
- cats
- pets
1. Introduction
As previously reported, the overuse of colistin has been shown to cause colistin resistance in bacteria colonizing the intestinal gut of animals [1]. However, only a few countries have prohibited the long-term use of colistin. Colistin reuse has resurfaced due to necessity and even more limited treatment options, particularly since the spread of multidrug-resistant bacteria (MDR) and carbapenem-resistant bacteria [2]. Colistin-resistant bacteria have become a significant public health issue. Indeed, colistin is a last-resort antibiotic; its failure to treat patients necessitates the development of new and more effective antibiotic therapies [3]. Since the discovery of the first plasmid harboring mcr-1 in China from pigs, the microbiota of animals appears as a source of colistin-resistant bacteria [4]. A variety of colistin-resistance gene variants have been discovered, ranging from mcr-2 to mcr-10 [5][6][7][8][9][10][11][12][13][5,6,7,8,9,10,11,12,13]. In 2016, mcr-1 was detected in several samples from humans and from food-producing animals on all seven continents and in more than 30 countries [14]. It is crucial to have a better understanding of the microorganisms found in pets, as well as the risk of pathogen transmission and antibiotic resistance genes such as mcr-1. mcr-1-producing bacteria have been reported in zoonotic transmission from pets to humans who adopt them for protection, entertainment, or companionship [15]. Close contact between cats, dogs, and their owners led to the occurrence and transmission of antibiotic-resistant microorganisms [16].
In Asia and the United States, mcr-1 in Enterobacteriaceae has already been reported in dogs and cats. The mcr genes in pets were also detected in Beijing, China, between 2012 and 2016 [17]. Further studies reported that dog feces in a city park in Quito (Ecuador) have a high prevalence of multidrug-resistant bacteria (MDR) [18]. Between 2017 and 2019, researchers in China reported an abundance of MDR such as Klebsiella pneumoniae harboring colistin resistance genes (mcr-1, mcr-8) and β-lactamases (blaOXA-181, blaNDM-5) in cats, which was significantly higher than in dogs [19]. In Europe, almost all colistin resistance studies have been focused in food-producing animals such as pigs and poultry [20][21][22][20,21,22]. Regarding Asia, for the first time, the mcr-1 gene was detected in dogs from South Korea, with an average nucleotide identity analysis similar to those found in Korean patients [23]. However, diseased dogs from Taiwan have Klebsiella spp and Enterobacter spp carrying mcr-1 gene [24].
Interestingly, colistin-resistant strains in pets have never been investigated in France, according to the literature. Colistin is currently not recommended for treating animal infections caused by Enterobacteriaceae in France unless it is necessary. Colistin is a critically important antibiotic that is used as a last resort treatment. Furthermore, because of unfavorable and toxic effects of colistin, its restriction has been requested by some agricultural or veterinary organizations and also by the European commission in 2016 [1]. Despite these precautions, the emergence of colistin resistance in animals is becoming more common, as evidenced by various studies on the subject. As reported, colistin resistance in animals is likely mediated by environmental factors and animal nutrition [25].
Colistin resistance in pets is rarely studied because the majority of reported studies were focused on food animals. However, pets are in daily close contact with humans. Determining the prevalence of colistin-resistant bacteria in pets is critical in order to identify any potential risk factors for colistin resistance transmission, particularly zoonotic transmission of bacteria [26].
2. Screening of Colistin-Resistant Bacteria in Pets
Among 157 collected fecal samples from domestic animals (dogs and cats), a total of 218 bacterial isolates were obtained from selective medium agar LBJMR (Vancomycin and colistin). As already reported, the gastrointestinal tract of animals can harbor a highly complex mixture of organisms that help maintain intestinal flora and overall health [27][31]. Indeed, the study of fecal samples depends upon the natural composition of the microorganisms and is specific to each animal. According to the literature, most studies have been performed on the general microorganism composition and most data have been derived from the analysis of feces from healthy laboratory animals [28][29][32,33]. In theiour study, the composition of microorganisms in the feces samples was described in terms of naturally colistin-resistant bacteria and those with acquired colistin resistance via mcr genes. Identification of colistin-resistant bacteria in cats revealed 150 bacteria, as represented in Figure 1. Identification encompassed 23 different bacterial species. Eighty percent (n = 120) of all identified bacteria were Gram-positive and 20% (n = 30) were Gram-negative. The dominant strains were naturally colistin-resistant for all GPB: Bacillus cereus, B. vallismortis, Enterococcus durans, E. faecalis, E. faecium, E. hirae, Leuconostoc citreum, L. mesenteroides, and Pediococcus pentosacens. A high proportion of Lactobacillus species were identified: L. curvatus, L. brevis, L. fructivorans, L. murinus, L. paracasei, L. planetarium, L. reuteri, L. sakei, and L. salivarius. In contrast, the GNB that were isolated were believed to have acquired resistance to colistin via mcr genes or other mechanisms. Here, 13% (n = 4) of colistin-resistant GNB were screened as being mcr carriers: E. coli (n = 3) and Rahnella aquatilis (n = 1). In addition, 87% (n = 26) of GNB strains were naturally resistant to colistin: Sphingomonas paucimobilis, Providencia heimbachae, and Hafnia alvei (Figure 1).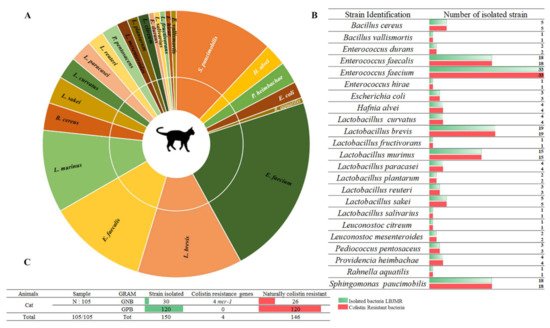
Figure 1. Proportion of colistin-resistant bacteria in feces of cats. (A). Pie chart representing the distribution of strains isolated in LBJMR. (B). Colistin-resistant bacteria verified by AST testing. (C). Statistical data on isolated bacteria according to Gram status and resistance mechanism.
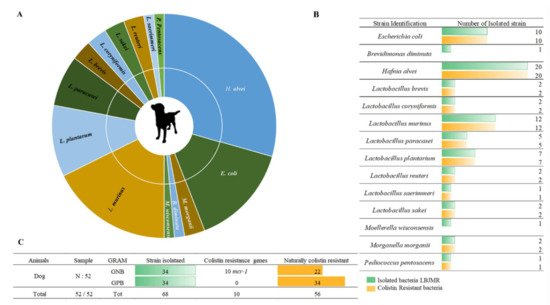
Figure 2. Illustration of colistin-resistant bacteria in the feces of dogs. (A). Distributed representation of colistin-resistant bacteria in a pie chart. (B). Distinguished colistin-resistant bacteria isolated in LBJMR. (C). Statistical analysis according to Gram status and colistin resistance.
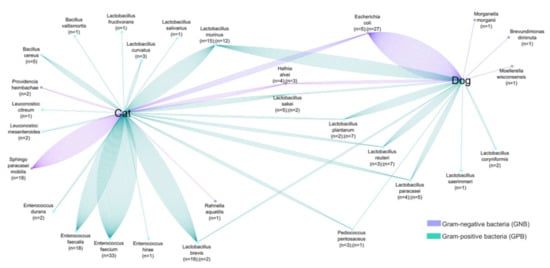
Figure 3. Comparative analyses of common colistin-resistant bacterial species found in the fecal samples of dogs and cats. The number of strains is proportional to the number of lines.
