Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Honghong Wu.
Nano-enabled agriculture is now receiving increasing attentions. Among the used nanomaterials, carbon-based nanomaterials are good candidates for sustainable agriculture. Previous review papers about the role of carbon-based nanomaterials in agriculture are either focused on one type of carbon-based nanomaterial or lack systematic discussion of the potential wide applications in agriculture.
- light conversion
- nanofertilizers
- nanopesticides
- nanosensors
- stress tolerance
- transgenic events
1. Introduction
According to an FAO report (FAO, Rome, Italy, 2020), the global production of primary crops in 2018 was 9.2 billion tons, which was about 50% higher than that in 2000. However, agricultural production is highly reliant on the use of agrochemicals such as fertilizer and pesticide, which is not sustainable. The use of pesticides and fertilizers reached 4.1 million tons and 188 million tons, respectively, in 2018 [1]. Over-use of fertilizers and pesticides not only increases the production cost of agricultural products, but also results in soil degradation and environmental pollution. Moreover, plant growth is accompanied by abiotic and biotic stresses. Thus, in addition to alleviating the heavy reliance on agrochemical applications, determining how to help plants face stress conditions is also important for efficient and sustainable agriculture. New strategies are required to address these issues to make agricultural production more efficient, resilient, and sustainable [2,3,4][2][3][4].
Nano-enabled agriculture is a rapidly growing field. Nanomaterials are materials with at least one dimension less than 100 nanometers, and have been shown to be promising in agriculture, especially in improving plant nutrition, reducing pests and diseases, improving stress tolerance, and testing the physiological state of plants. Among these materials, carbon-based nanomaterials (CNMs) are commonly used. The CNM family mainly includes carbon dots (CDs), carbon nanotubes (CNTs), carbon fullerenes (C60), graphene (GRA), graphene oxide (GO), nanohorns (CNHs), and carbon nanofibers (CNFs) [5]. The use of CNMs to improve plant stress tolerance, and thus agricultural production, is well known. For example, 10 mg/L putrescine-functionalized CDs has been used to improve grape salt tolerance [6]. Reduced GO (rGO) showed obvious inhibitory effects on Fusarium graminearum and Fusarium poae in vitro [7]. Although good reviews have been published on the use of carbon-based nanomaterials in agriculture [8], they are either focused on one type of carbon-based nanomaterial or do not mention/discuss the use of carbon-based nanomaterials in light converters, nanosensors, and delivery tools. In this review, wThe researchers mainly focus on discussing the role of CNMs in nanosensors, agrochemical delivery, and light converters in agriculture (Figure 1). WThe researchers hope this review can benefit the communication between the plant nanobiotechnology community and the agricultural production-related field, and can encourage people to explore more carbon-based nanomaterials for agricultural application. For more details regarding the phytotoxicity of CNMs, please refer to previous papers [9].
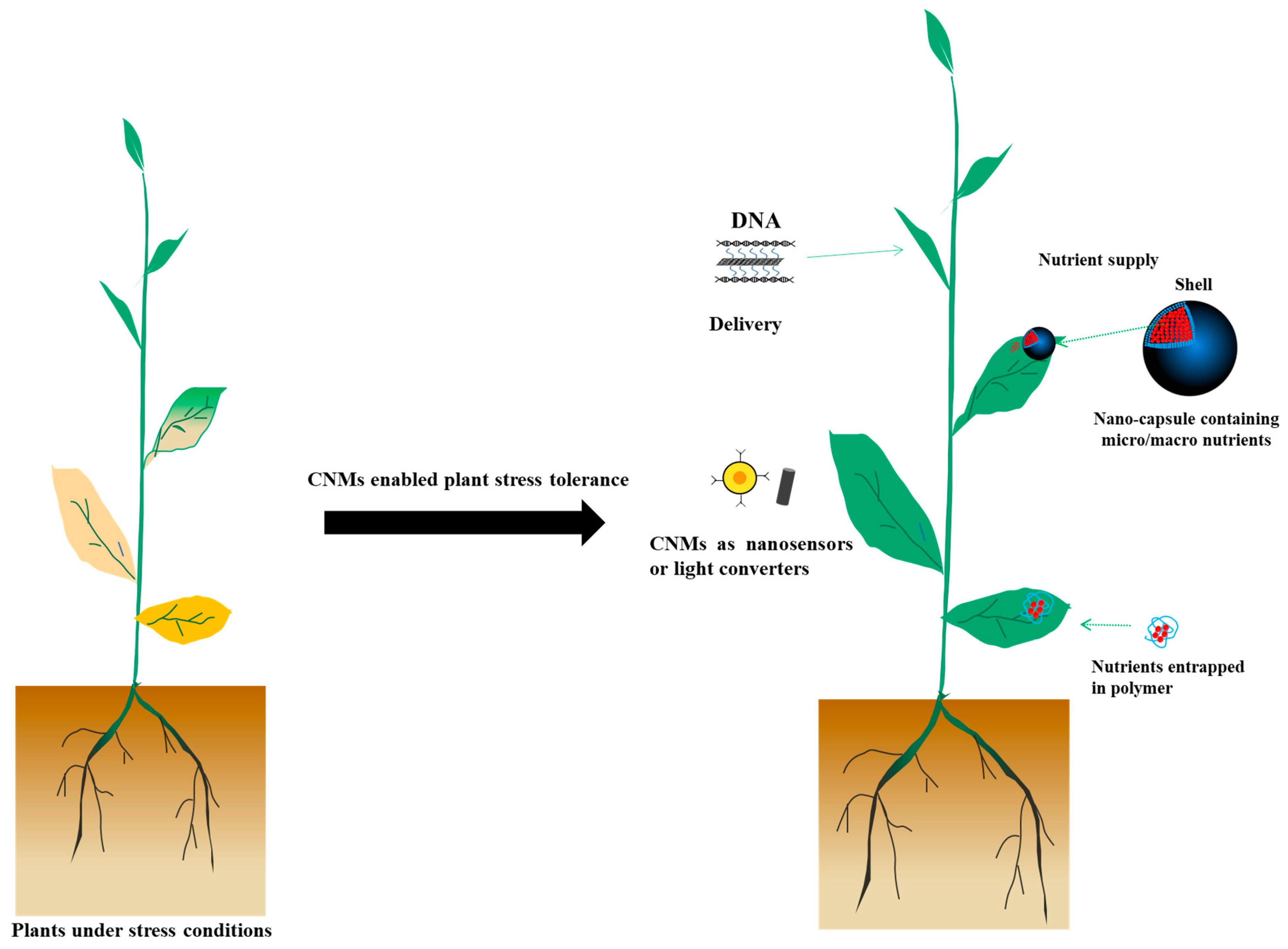
Figure 1.
The role of CNMs in nanosensors, agrochemical delivery, and light converters in agriculture.
2. CNMs as Nanosenors to Detect Stress Signaling Molecules and Pesticide Residues
2.1. CNMs as Nanosensors to Detect Signaling Molecules in Plants under Abiotic and Biotic Stress
Against the background of climate change and global warming, the frequency and degree of plant-stress-related events have increased over time [10]. To enable sustainable agriculture, remote sensing technology was developed to monitor and manage plant stress conditions to alleviate the stress that results in the reduction in crop yield and quality [11]. Traditional remote sensing sensors mainly reveal stress through the changes in the plant phenotype and physiological characteristics during stress, which are mainly the accumulative traits [12]. This approach lacks the ability to detect the changes in chemical signaling molecules in plants upon the onset of stress. Although fluorescent dyes can be used to label signaling molecules, they have issues associated with photo bleaching, and thus cannot support long-term real-time monitoring [13]. By comparison, nanosensors may be a good candidate for monitoring chemical signaling molecules in plants during long-term and real-time tracking. Nanosensors can be used to monitor crop maturity and health, and to detect fertilizers, pesticides, and moisture in the soil, and thus can help to make quick decisions to benefit agricultural production [14]. Emerging optical nanosensors can directly detect chemical signals inside plants, providing enough time for imaging devices to detect signals and indicating the need to improve plant growth conditions [15,16][15][16].
Among nanosensors, CNTs are widely used to detect signaling molecules such as H2O2 [17], Ca2+ [18], and NO [19] in plants under abiotic and biotic stress, with the advantages of good fluorescence stability, long life, and emitting fluorescence in the relatively transparent near-infrared emission spectrum region of living tissues [20]. For example, the near-infrared fluorescence emitted by CNTs can be quenched by H2O2 within the physiological range of 10 to 100 μM in plants, which can be used to remotely report plant stress status without causing mechanical leaf damage. Due to the sensitivity of 10 μM H2O2, hemin complexed-DNA aptamer conjugated SWCNTs were developed for real-time monitoring of H2O2 content in leaves under UV, high light, and Flg22 stress [16]. This showed that the profile of leaf H2O2 content changes vary among the stresses, indicating that hemin complexed-DNA aptamer conjugated SWCNTs may be a good candidate to enable early stress detection in plants. In addition, SWCNTs can be combined with a hemoglobin (Hb)-modified carbon fiber ultramicroelectrode (CFUME) to construct an in vivo H2O2 sensor based on direct electron transfer. This Hb/SWCNTs/CFUME sensor can detect a dynamic range of up to 0.405 mM H2O2 and has a low detection limit of 4 μM H2O2 at a low working potential of −0.1 V [21]. For monitoring plant diseases such as Huanglong disease in citrus, SWCNTs functionalized with anti SDE1 (Sec-Delivered Effector 1) polyclonal antibodies can detect the SDE1 secreted protein, a biomarker of Huanglong disease, with high sensitivity and high specificity. This overcomes the traditional Huanglong disease nucleic acid and other problems of low diagnostic accuracy [22]. Single-stranded DNA-functionalized SWCNTs can also be used to detect volatile markers such as ethylhexanol, linalool, tetradecene, and phenylacetaldehyde of Huanglong disease, which is lethal to citrus, so as to realize the monitoring of the asymptomatic stage of the disease [23]. Moreover, 6-ssDNA modified SWCNTs can rapidly detect 4-ethylphenol, a major volatile organic compound released by Phytophthora cactorum-infected strawberries, within a few minutes via an electrostatic gating mechanism, with a wide concentration range, and high detection sensitivity and recovery [24].
Overall, these CNT-based nanosensors show potential in monitoring stress signaling molecules to better allow early detection of plant stress. However, to date, these studies have mostly been conducted in laboratories, and the proposed approaches have not been tested under actual agricultural conditions. Whether the performance of these CNT-based nanosensors will be affected by factors such as temperature (heat, cold, and freezing), wind, rain, light (high light, shading, and UV), and aerial pollutants is still unknown. It is also necessary to conduct comprehensive analysis and research on the connection between nanosensor-based sensing, plant stress detection, data storage and analysis, and agricultural equipment. Moreover, the current studies of nanosensors used in stress detection are mainly focused on the detection of stress-related plant signaling molecules, with less attention on monitoring plant nutrient deficiencies, which are also an important factor limiting agricultural production.
2.2. CNMs as Sensors to Detect Pesticide Residues
Pesticides enable the negative impacts of weeds and pests on crop production to be prevented and controlled, which is essential in agricultural production. However, the excessive use of pesticides leads to the release of a large number of these compounds into the environment and ecosystem. The remaining pesticide and its residues seriously endanger food safety, the ecological environment, and human health [25]. Traditional pesticide detection strategies, such as chromatography, electrophoresis, and mass spectrometry, are usually not suitable for the real-time and long-term detection of small amounts of pesticide residues and for performing on-site detection [26]. Although these methods are sensitive and accurate, they have the disadvantages of being time-consuming, and requiring complex sample preparation and expensive instrumentation. Therefore, the use of nanosensors for rapid analysis of pesticide residues may be an effective strategy. Compared with traditional methods, nanosensors have the advantages of simplicity, high sensitivity, strong selectivity, and direct on-site detection [27,28][27][28].
Nanosensors based on CNMs with autofluorescence have been widely used in pesticide detection [29]. Pesticides can be identified by quenching the autofluorescence of CDs. For example, diazinon, glyphosate, and semicarbazide residues in cherry tomatoes can be detected by CDs’ fluorescent sensors, with detection limits as low as 0.25, 0.5, and 2 ng/mL, respectively, which are the lowest detection limits of typical detection methods such as chromatography [30]. The broad-spectrum organophosphorus insecticidal malathion can also be recognized by a sensor composed of carbon dots and gold nanoparticles. The gold nanoparticles quench the fluorescence of the carbon dots based on the principle of fluorescence resonance energy transfer, but the fluorescence is detected after the detection of malathion. The intensity increases and the color changes from red to blue; thus, it is possible to quickly detect malathion through simple visual judgment [31]. Another good candidate for detection of pesticide residues is graphene quantum dots, which are widely used in biological imaging and optical sensing due to their low cytotoxicity and excellent biocompatibility [32]. For example, nitrogen-doped graphene quantum dots can be used to prepare test strip sensors for pesticides [33]. By forming a uniform PDA molecularly imprinted polymer film on the surface of a nitrogen-doped graphene quantum dot strip, thiacloprid can be specifically recognized and detected, with the detection limit as low as 0.1–4 mg/L. In addition to graphene quantum dots, graphene can also be used to prepare sensors to detect pesticides. For example, by inhibiting the peroxidase activity of graphene, aromatic pesticides can be detected by graphene doped with heteroatoms, which shows the changes in the color reaction of hydrogen peroxide and tetramethylbenzidine dihydrochloride. It is suggested that different element-doped graphene sensors can be designed to detect different aromatic pesticides [34]. Polydopamine-functionalized graphene nanozymes can effectively detect triazine pesticides by changing the activity of peroxidase through pesticides [35].
In summary, CNM-based nanosensors are good candidates for monitoring pesticide residues to benefit agricultural production. More attention on developing environmentally friendly and biocompatible CNM-based nanosensors to detect single or mixed pesticide residues is encouraged. For the use of CNMs for heavy metal detection, please refer to previous review papers [36].
3. CNMs as a Tool to Deliver Agrochemicals and Functional Genetic Materials
3.1. Use of CNMs to Deliver Agrochemicals
Use of agrochemicals, such as fertilizer and pesticide, is essential for agricultural production. However, the utilization rate of most chemical fertilizers in plants is less than 50% [37], which not only limits efficient agricultural production but also results in environment pollution. Most fertilizers are water-soluble and are easily leached in soil, resulting in environmental pollution and increased costs due to possible multiple applications of fertilizers [38]. Compared with their conventional counterparts, the efficacy gain of nanofertilizers is 18–29% higher [39]. Nanoparticles containing one or more elements required for plant growth can be directly used as nanofertilizers [40,41,42,43][40][41][42][43]. As a carrier to enable slow and/or targeted delivery of nutrients into plants, nanomaterials can also be used as nanofertilizers [44]. Regarding the use of nanofertilizers in agriculture, please refer to previous good reviews [45,46][45][46]. Here, the weresearchers mainly focus on CNMs as nanofertilizers in agriculture.
Having the advantages of stable molecular structure, good biocompatibility, less toxicity, and uniform dispersion in application medium, CNMs can be used as good fertilizer carriers. For example, GO nanomaterials are good carriers of trace elements [47]. The oxygen-containing groups on its surface can electrostatically adsorb trace elements and also have two-phase release characteristics, showing quick release at the early stage, and then slow and continuous release. Indeed, GO sheets are able to deliver Zn and Cu elements more efficiently in wheat than zinc or copper salts [44]. In addition to GO, negatively charged copper CNF can also be used as a slow-release carrier of micronutrient copper [48]. CNF-Cu can transfer from roots to shoots through xylem and slowly release copper in plants, showing significantly improved water absorption capacity, germination rate, root/shoot ratio, and protein content of chicory. Negatively charged fullerenol can be used as a leaf slow-release fertilizer to promote the absorption of Fe2+ in cucumber leaves and to alleviate the symptoms of iron deficiency [49]. Furthermore, previous research showed that adding nanocarbon to slow-release fertilizer can significantly improve rice yield and nitrogen use efficiency, and reduce nitrogen loss, indicating that nanocarbon can be used as an environment-friendly slow-release fertilizer coating material [50]. These results show that CNMs can be good carriers to deliver nutrients to plants or to improve the effect of fertilizers.
In addition to fertilizers, pesticides are another major component of agrochemicals for agriculture. However, public concerns exist regarding the biosafety and pollution issues of traditional pesticides due to their easy leaching, volatilization, and loss properties [50]. Excessive use of pesticides has also caused many problems that need to be addressed urgently, such as plant disease resistance, destruction of soil biodiversity, and adverse effects on human health and the environment [51]. Therefore, more efficient and environmentally friendly solutions regarding the use of pesticides are encouraged. Nano-pesticides (including nano-insecticides, nano-herbicides, and nano-fungicides) can reduce volatilization and degradation of pesticides, improve utilization efficiency, reduce the use of pesticides, and alleviate environmental risks [52,53][52][53]. In addition to adsorbing harmful organic matter to reduce the solubility and bioavailability of organic matter, CNMs are promising materials that can be used as a pesticide carrier to improve the utilization efficiency of pesticides [54,55,56,57][54][55][56][57]. Currently, due to the unique physical and chemical properties mentioned above, GO is one of the most widely used carriers in the field of nano-pesticides. For example, rGO has the advantages of high pesticide adsorption capacity (up to 1200 mg/g for chloropyrifos), low toxicity, good antibacterial performance, insensitivity to pH value change, and the ability to be reused [57]. GO loaded with red spider insecticide can be completely adsorbed on the surface of the red spider and have a strong toxic effect [58]. GO can carry different types of pesticides through surface modification. Tong et al. (2018) used polydopamine-modified GO as the carrier of water-soluble pesticides, which alleviated the issue of easy loss of water-soluble pesticides, enabled controlled release of pesticides, enhanced the adhesion between pesticides and plants, and thus improved the utilization efficiency [59]. Hydrophobic pesticides can be loaded by polylactic acid-modified GO [60]. However, GO also has disadvantages, such as low stability under acidic solution [61]. Song et al. (2019) developed nano-biochar as the carrier of emamectin benzoate, and used carboxymethyl chitosan as the pH-responsive switch to control the delivery and release of emamectin benzoate. As a result, the water solubility, dispersion stability, and UV resistance of the delivery system were significantly improved, ensuring its long-term control of pests [62]. In addition to GO, CNTs can also be used as a sustained-release system for pesticides. For example, MWCNTs grafted with polycitric acid (PCA) can deliver zineb, an antifungal pesticide. Compared with zineb in bulk, the novel CNT-PCA-Zineb hybrid material has better water solubility, higher stability, and stronger toxicity to Alternaria [63].
Overall, CNMs are good candidates to deliver agrochemicals into plants with better efficiency than conventional fertilizers and pesticides. However, there is an urgent need to understand how plants respond to their exposure. Moreover, the addition of CNMs has increased the complexity of the agro-ecosystem; whether they represent a new pollutant or a new opportunity is discussed in detail by Kah [64]. With regard to the future of nano-agrochemicals, it is necessary to fully consider the views in many fields of science, industry, and regulation, so that the agrochemicals sector can make use of nanotechnology and reduce its negative impact on human beings and the environment as much as possible.
3.2. Use of CNMs to Deliver Functional Genetic Materials
To feed an expected population of over 9 billion in 2050, the breeding of stress-tolerant species is of importance to ensure the food supply in the near future. At present, the commonly used transformation methods in plants, such as gene gun bombardment and agrobacterium-mediated transformation, have problems such as restriction of the host type, restriction of transformation efficiency by the cell wall, damage to the plant tissue, and pathogenicity [65]. In recent years, nano-enabled transgenic events have shown convincing progress. Among the nanomaterials used, carbon nanotubes and carbon dots are important. Compared with agrobacterium-mediated gene transformation, nanomaterials can deliver nucleic acids faster. In addition, some auto-fluorescent CNMs, such as CDs, can form relatively small complexes with nucleic acids and deliver the nucleic acids into cells, and track the complexes directly and conveniently [65,66,67][65][66][67]. A PEI-modified CD-siRNA delivery system has been developed to deliver and silence target genes in tobacco and tomato [68]. In addition to CDs, CNTs are the most widely tested carriers for nano-enabled transgenic events. Demirer et al. (2019) established a method for delivering plasmid DNA with PEI-modified SWCNTs, which were able to effectively deliver plasmid DNA to wheat and cotton without transgene integration, and showing highly expressed YFP protein [69]. Further studies showed that SWCNTs can also deliver siRNA to mature plant leaves to achieve instantaneous gene silencing, with a gene knockout efficiency as high as 95% [70]. In addition, SWCNTs modified with chitosan can achieve chloroplast genetic transformation without external biological or chemical assistance, which is much cheaper and simpler than conventional methods [71]. Researchers also developed a system of SWCNTs and conventional cell-shuttle peptides to improve the efficiency of targeted peptide–plasmid transformation [72]. Overall, CNMs play an important role in nano-enabled transgenic events and may be good candidate for targeted delivery of functional materials. However, the plant transformation method based on CNMs is still at the embryonic stage and needs to be further explored.
4. CNMs as a Light Converter for Augmenting Plant Photosynthesis
Plants convert solar energy into chemical energy through chloroplast photosynthesis [73,74][73][74]. However, the utilization rate of sunlight by chloroplasts is less than 10%, and is limited to the visible spectral range (400–700 nm), mainly in the blue and red regions [75,76][75][76]. UV and nIR lights are not utilized for plant photosynthesis. Therefore, to expand the light spectrum for plant photosynthesis, developing high-performance light conversion materials with blue and red broadband emissions to make maximum use of solar energy may be a feasible approach [77,78,79,80][77][78][79][80].
4.1. CNMs as a Down-Conversion Light Converter
UV light (200–400 nm) induces the generation of reactive oxygen species in plants and negatively affects agricultural production [81]. In order to increase plant photosynthesis, various light-trapping nanomaterials have been used to convert poorly absorbed ultraviolet light into highly absorbed visible light to improve the conversion efficiency of the light used by chloroplasts [82]. Due to their stable emission and adjustability of the photoluminescence spectrum, CNMs are widely used as down-conversion nanomaterials (DCNMs) to convert ultraviolet (UV) light into photosynthetic active radiation [83,84,85,86,87][83][84][85][86][87]. Most CDs synthesized at present can emit blue fluorescence under UV excitation [88,89,90][88][89][90]. Vinyl alcohol-encapsulated CDs converted UV to blue light and enhanced photosynthetic efficiency in lettuce [91]. Amine-functionalized CDs can be strongly conjugated on chloroplast surfaces and promote photosynthesis by accelerating the conversion of solar energy [92]. Li et al. (2018) designed a new dual-wavelength luminescent CD that exhibits strong absorption in the UV light region and emits light that exactly matches the chloroplast absorption spectrum (blue and red light) [93]. The adenosine triphosphate produced by the hybrid photosystem (chloroplast coating with CDs) in vitro is 2.8 times that of the chloroplast itself. In the in vivo experiments, the electron transfer rate of the Rome lettuce leaves coated with CDs increased by 25% at the maximum. It should be noted that the impact of CDs on plant photosynthesis may be affected by the quantum yield. For example, chloroplasts can only use the blue fluorescence re-emitted by CDs with medium QY (46.42%) to enhance photosynthesis, but not low and high quantum yield CDs [94]. This suggests that properties such as the emission intensity, quantum yield, and emission light spectrum of CNMs used for converting UV to visible light should be properly designed before being applied to plants. Moreover, as a UV-visible light color converter, CDs can also be applied to plastic films and LEDs for greenhouse to promote plant growth [95,96,97,98][95][96][97][98].
4.2. CNMs as an Up-Conversion Light Converter
nIR light accounts for about 52% of the solar spectrum, but cannot be used by plants, resulting in a serious waste of solar light resources [99]. Up-conversion nanomaterials (UCNMs) can convert nIR light to visible light that can be utilized by plants. The conversion of nIR to visible light by UCNMs is a nonlinear optical process, which absorbs two or more low-energy photons from nIR light and converts them into high-energy photons having a shorter wavelength and stronger energy via energy transfer, excited state absorption, or multiphoton absorption [100]. Carbon nanomaterials can work with up-conversion optical materials to promote nIR light conversion to enhance plant photosynthesis [101,102][101][102]. Doping CDs into the up-conversion material NaYF4: Yb, Er, CDs can shift the green emission of NaYF4: Yb, Er to red light [103]. Mung beans sprayed with NaYF4: Yb, Er@CD nanocomposite showed a significantly increased photosynthetic rate [103]. It is argued that CNMs can also be used as up-conversion nanomaterials (UCNMs). However, to date, CNMs as UCNMs have rarely been directly applied to the improvement in plant photosynthetic efficiency.
In summary, the use of CNMs to convert UV and nIR to visible light may be a potential means to augment plant photosynthesis. However, to date, the leaf and/or root application of CNMs as light conversion materials to improve plant growth is still mainly at the proof-of-concept stage. Moreover, to facilitate the use of CNMs as a light converter in plants, addition factors need to be considered, such as (1) the biocompatibility, long-term safety, and toxicity of light converting CNMs in plants; (2) the light conversion efficiency; and (3) the heat generated during CNM-enabled light conversion in plants. For example, after nIR dyes are attached to the surface of lanthanide-doped UCNPs, the up-conversion efficiency is more than 30,000 times higher than that of UCNPs alone [104]. Moreover, some optical nanomaterials, such as carbon nanohorns (CNHs) and gold nanorods (AuNRs), generate local heat through photothermal conversion under nIR laser irradiation [105,106][105][106].
References
- FAO Food. World Food and Agriculture Statistical Yearbook; FAO-Food: Rome, Italy, 2020.
- National Academies of Sciences, Engineering, and Medicine. Science Breakthroughs to Advance Food and Agricultural Research by 2030; National Academies Press: Washington, DC, USA, 2019.
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522.
- Pretty, J. Intensification for redesigned and sustainable agricultural systems. Science 2018, 362, eaav0294.
- Gogotsi, Y.; Volker, P. Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2006.
- Gohari, G.; Panahirad, S.; Sadeghi, M. Putrescine-functionalized carbon quantum dot (put-CQD) nanoparticles effectively prime grapevine (Vitis vinifera cv. ‘Sultana’) against salt stress. BMC Plant Biol. 2021, 21, 120.
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806.
- Li, Y.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. A review on the effects of carbon dots in plant systems. Mater. Chem Front. 2020, 4, 437–448.
- Xiao, A.; Wang, C.; Chen, J.; Guo, R.; Yan, Z.; Chen, J. Carbon and Metal Quantum Dots toxicity on the microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2016, 133, 211–217.
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377.
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 2022, 10, 1–12.
- Mahlein, A.K. Plant Disease Detection by Imaging Sensors-Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2016, 100, 241–251.
- Fichman, Y.; Miller, G.; Mittler, R. Whole-Plant Live Imaging of Reactive Oxygen Species. Mol. Plant 2019, 12, 1203–1210.
- Antonacci, A.; Arduini, F.; Moscone, D.; Palleschi, G.; Scognamiglio, V. Nanostructured (Bio)sensors for smart agriculture. Trac-Trend Anal. Chem. 2018, 98, 95–103.
- Giraldo, J.P.; Wu, H.; Newkirk, G.M.; Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553.
- Wu, H.; Nißler, R.; Morris, V.; Herrmann, N.; Hu, P.; Jeon, S.J.; Kruss, S.; Giraldo, J.P. Monitoring Plant Health with Near-Infrared Fluorescent H2O2 Nanosensors. Nano Lett. 2020, 20, 2432–2442.
- Huang, X.; Zhang, J.; Zhang, L.; Su, H.; Liu, X.; Liu, J. A sensitive H2O2 biosensor based on carbon nanotubes/tetrathiafulvalene and its application in detecting NADH. Anal. Biochem. 2020, 589, 113493.
- Gao, J.; Wang, L.; Kang, S.G.; Zhao, L.; Ji, M.; Chen, C.; Zhao, Y.; Zhou, R.; Li, J. Size-dependent impact of CNTs on dynamic properties of calmodulin. Nanoscale 2014, 6, 12828–12837.
- Song, H.; Li, K.; Wang, C. Selective Detection of NO and NO2 with CNTs-Based Ionization Sensor Array. Micromachines 2018, 9, 354.
- Boghossian, A.A.; Zhang, J.; Barone, P.W.; Reuel, N.F.; Kim, J.H.; Heller, D.A.; Ahn, J.H.; Hilmer, A.J.; Rwei, A.; Arkalgud, J.R.; et al. Near-infrared fluorescent sensors based on single-walled carbon nanotubes for life sciences applications. ChemSusChem 2011, 4, 848–863.
- Ren, Q.Q.; Yuan, X.J.; Huang, X.R.; Wen, W.; Zhao, Y.D.; Chen, W. In vivo monitoring of oxidative burst on aloe under salinity stress using hemoglobin and single-walled carbon nanotubes modified carbon fiber ultramicroelectrode. Biosens. Bioelectron. 2013, 50, 318–324.
- Tran, T.T.; Clar, K.; Ma, W.; Mulchandani, A. Detection of a secreted protein biomarker for citrus Huanglongbing using a single-walled carbon nanotubes-based chemiresistive biosensor. Biosens. Bioelectron. 2020, 147, 111766.
- Wang, H.; Ramnani, P.; Pham, T.; Villarreal, C.C.; Yu, X.; Liu, G.; Mulchandani, A. Gas Biosensor Arrays Based on Single-Stranded DNA-Functionalized Single-Walled Carbon Nanotubes for the Detection of Volatile Organic Compound Biomarkers Released by Huanglongbing Disease-Infected Citrus Trees. Sensors 2019, 19, 4795.
- Wang, H.; Wang, Y.; Hou, X.; Xiong, B. Bioelectronic Nose Based on Single-Stranded DNA and Single-Walled Carbon Nanotube to Identify a Major Plant Volatile Organic Compound (p-Ethylphenol) Released by Phytophthora Cactorum Infected Strawberries. Nanomaterials 2020, 10, 479.
- Agarwal, R. The International Code of Conduct on Distribution and Use of Pesticides. In Chemicals, Environment, Health; CRC Press: Boca Raton, FL, USA, 2011; pp. 211–226.
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials–a review. Anal. Chim. Acta 2016, 945, 9–22.
- Dutta, R.R.; Puzari, P. Amperometric biosensing of organophosphate and organocarbamate pesticides utilizing polypyrrole entrapped acetylcholinesterase electrode. Biosens. Bioelectron. 2014, 52, 166–172.
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Song, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109.
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens. Bioelectron. 2017, 94, 292–297.
- Ashrafi, T.F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646.
- Liang, N.; Hu, X.; Li, W.; Mwakosya, A.W.; Guo, Z.; Xu, Y.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; et al. Fluorescence and colorimetric dual-mode sensor for visual detection of malathion in cabbage based on carbon quantum dots and gold nanoparticles. Food Chem. 2021, 343, 128494.
- Yuan, H.; Yu, J.; Feng, S.; Gong, Y. Highly photoluminescent pH-independent nitrogen-doped carbon dots for sensitive and selective sensing of p-nitrophenol. RSC Adv. 2016, 6, 15192–15200.
- Liu, Y.; Cao, N.; Gui, W.; Ma, Q. Nitrogen-doped graphene quantum dots-based fluorescence molecularly imprinted sensor for thiacloprid detection. Talanta 2018, 183, 339–344.
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme sensor arrays based on heteroatom-doped graphene for detecting pesticides. Anal. Chem. 2020, 92, 7444–7452.
- Boruah, P.K.; Darabdhara, G.; Das, M.R. Polydopamine functionalized graphene sheets decorated with magnetic metal oxide nanoparticles as efficient nanozyme for the detection and degradation of harmful triazine pesticides. Chemosphere 2021, 268, 129328.
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.H. Carbon dots as an effective fluorescent sensing platform for metal ion detection. Nanoscale Res. Lett. 2019, 14, 272.
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91.
- Bandyopadhyay, S.; Ghosh, K.; Varadachari, C. Multimicronutrient slow-release fertilizer of zinc, iron, manganese, and coppe. Int. J. Chem. Eng. 2014, 2014, 327153.
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684.
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci Technol. 2013, 47, 10645–10652.
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559.
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 2019, 8, 355.
- Ye, Y.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotoxicol. Environ. Saf. 2019, 184, 109671.
- Kabiri, S.; Degryse, F.; Tran, D.N.; da Silva, R.C.; McLaughlin, M.J.; Losic, D. Graphene oxide: A new carrier for slow release of plant micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325–43335.
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and nanofertilizers for sustainable agriculture: Phycoprospects and challenges. Sci. Total Environ. 2022, 803, 49990.
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270.
- Zhou, D.D.; Jiang, X.H.; Lu, Y.; Fan, W.; Huo, M.X.; Crittenden, J.C. Cotransport of graphene oxide and Cu (II) through saturated porous media. Sci. Total Environ. 2016, 550, 717–726.
- Ashfaq, M.; Verma, N.; Khan, S. Carbon nanofibers as a micronutrient carrier in plants: Efficient translocation and controlled release of Cu nanoparticles. Environ. Sci.-Nano 2017, 4, 138–148.
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N. Fullerenol increases effectiveness of foliar iron fertilization in iron-deficient cucumber. PLoS ONE 2020, 4, e0232765.
- Mew, E.J.; Padmanathan, P.; Konradsen, F.; Eddleston, M.; Chang, S.S.; Phillips, M.R.; Gunnell, D. The global burden of fatal self-poisoning with pesticides 2006-15: Systematic review. J. Affect. Disord. 2017, 219, 93–104.
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 124657.
- Mohamed, T.; ALmoshadak, A.S.; Shafi, M.E.; Albaqami, N.M.; Saad, A.M.; El-Tahan, A.M.; Desoky, E.M.; Elnahal, A.S.; Almakas, A.; El-Mageed, T.A.; et al. Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review. Saudi J. Biol. Sci. 2021, 28, 7349–7359.
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67.
- Pan, B.; Xing, B. Manufactured nanoparticles and their sorption of organic chemicals. Adv. Agron. 2010, 108, 137–181.
- Chen, G.C.; Shan, X.Q.; Pei, Z.G.; Wang, H.; Zheng, L.R.; Zhang, J.; Xie, Y.N. Adsorption of diuron and dichlobenil on multiwalled carbon nanotubes as affected by lead. J. Hazard. Mater. 2011, 188, 156–163.
- Fan, X.; Xu, J.; Lavoie, M.; Peijnenburg, W.J.G.M.; Zhu, Y.; Lu, T.; Fu, Z.; Zhu, T.; Qian, H. Multiwall carbon nanotubes modulate paraquat toxicity in Arabidopsis thaliana. Environ. Pollut. 2018, 233, 633–641.
- Maliyekkal, S.M.; Sreeprasad, T.S.; Krishnan, D.; Kouser, S.; Mishra, A.K.; Waghmare, U.V.; Pradeep, T. Graphene: A reusable substrate for unprecedented adsorption of pesticides. Small 2013, 9, 273–283.
- Wang, X.; Xie, H.; Wang, Z.; He, K. Graphene oxide as a pesticide delivery vector for enhancing acaricidal activity against spider mites. Colloid Surf. B Biointerfaces 2019, 173, 632–638.
- Tong, Y.; Shao, L.; Li, X.; Lu, J.; Sun, H.; Xiang, S.; Zhang, Z.; Wu, Y.; Wu, X. Adhesive and Stimulus-Responsive Polydopamine-Coated Graphene Oxide System for Pesticide-Loss Control. J. Agric. Food Chem. 2018, 66, 2616–2622.
- Wang, Y.; Li, C.; Wang, T.; Li, X.; Li, X. Polylactic Acid-Graphene Oxide-based Materials for Loading and Sustained Release of Poorly Soluble Pesticides. Langmuir 2020, 36, 12336–12345.
- Yeh, C.N.; Raidongia, K.; Shao, J.; Yang, Q.H.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2014, 7, 166–170.
- Song, S.; Wang, Y.; Xie, J.; Sun, B.; Zhou, N.; Shen, H.; Shen, J. Carboxymethyl Chitosan Modified Carbon Nanoparticle for Controlled Emamectin Benzoate Delivery: Improved Solubility, pH-Responsive Release, and Sustainable Pest Control. ACS Appl. Mater. Interfaces 2019, 11, 34258–34267.
- Sarlak, N.; Taherifar, A.; Salehi, F. Synthesis of nanopesticides by encapsulating pesticide nanoparticles using functionalized carbon nanotubes and application of new nanocomposite for plant disease treatment. J. Agric. Food Chem. 2014, 62, 4833–4838.
- Kah, M. Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation? Front. Chem. 2015, 3, 64.
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042.
- Wang, H.; Koleilat, G.I.; Liu, P.; Jiménez-Osés, G.; Lai, Y.C.; Vosgueritchian, M.; Bao, Z. High-yield sorting of small-diameter carbon nanotubes for solar cells and transistors. ACS Nano 2014, 8, 2609–2617.
- Pierrat, P.; Wang, R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichle, R.A.; Pons, F.; Lebeau, L. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers. Biomaterials 2015, 51, 290–302.
- Schwartz, S.H.; Hendri, X.B.; Hoffe, R.P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657.
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon nanotube-mediated DNA delivery without transgene integration in intact plants. Nat. Protoc. 2019, 14, 2954–2971.
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, e0495.
- Kwak, S.Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455.
- Golestanipour, A.; Nikkhah, M.; Aalami, A.; Hosseinkhani, S. Gene Delivery to Tobacco Root Cells with Single-Walled Carbon Nanotubes and Cell-Penetrating Fusogenic Peptides. Mol. Biotechnol. 2018, 60, 863–878.
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774.
- Shevela, D.; Bjorn, L.O. Photosynthesis: Solar Energy for Life; World Scientific Publishing: Singapore, 2018.
- Pessarakli, M. Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018.
- Duan, P.; Huang, T.; Xiong, W.; Shu, L.; Yang, Y.; Shao, C.; Tang, R. Protection of photosynthetic algae against ultraviolet radiation by one-step CeO2 shellization. Langmuir 2017, 33, 2454–2459.
- Wang, Y.; Xie, Z.; Wang, X.; Peng, X.; Zheng, J. Fluorescent carbon-dots enhance light harvesting and photosynthesis by overexpressing PsbP and PsiK genes. J. Nanobiotechnol. 2021, 19, 260.
- Liu, X.Y.; Jiao, X.L.; Chang, T.T.; Guo, S.R.; Xu, Z.G. Photosynthesis and leaf development of cherry tomato seedlings under different LED-based blue and red photon flux ratios. Photosynthetica 2018, 56, 1212–1217.
- Ren, J.; Zhou, X.; Wang, Y. Dual-emitting ZJU-28 (X = Cl, Br, I) composites with enhanced stability and unique optical properties for multifunctional applications. Chem. Eng. J. 2020, 391, 123622.
- Liang, L.; Mei, L.; Liu, H.; Wang, C.; Liao, L. Intense broad-band absorption and blue-emitting Ca9La (PO4)5 (SiO4)Cl2: Eu2+ phosphor under near-ultraviolet excitation. J. Lumin. 2019, 206, 154–157.
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-induced cell death in plants. Int. J. Mol. Sci. 2013, 14, 1608–1628.
- Wang, Y.; Li, S.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles to augment photosynthesis of chloroplasts. Angew. Chem. Int. Ed. 2017, 56, 5308–5311.
- Choi, Y.; Kwon, O.H.; Kim, B.S. Carbon Dots: Bottom-Up Syntheses, Properties, and Light-Harvesting Applications. Chem. Asian J. 2018, 3, 586–598.
- Li, Y.; Xu, X.; Lei, B.; Zhuang, J.; Zhang, X.; Hu, C.; Cui, J.; Liu, Y. Magnesium-nitrogen co-doped carbon dots enhance plant growth through multifunctional regulation in photosynthesis. Chemical Engineer. J. 2021, 442, 130114.
- Song, H.; Liu, X.; Wang, B.; Tang, Z.; Lu, S. High production-yield solid-state carbon dots with tunable photoluminescence for white/multi-color light-emitting diodes. Sci. Bull. 2019, 64, 1788–1794.
- Wang, B.; Li, J.; Tang, Z.; Yang, B.; Lu, S. Near-infrared emissive carbon dots with 33.96% emission in aqueous solution for cellular sensing and light-emitting diodes. Sci. Bull. 2019, 64, 1285–1292.
- Qian, T.; Zhu, S.; Wang, H.; Li, A.; Fan, B. Comparative study of single-walled carbon nanotubes and graphene nanoplatelets for improving the thermal conductivity and solar-to-light conversion of PEG-infiltrated phase-change material composites. ACS Sustain. Chem. Eng. 2018, 7, 2446–2458.
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Xiong, D.; Lee, Y.R. Facile hydrothermal synthesis of nitrogen rich blue fluorescent carbon dots for cell bio-imaging of Candida albicans. Process. Biochem. 2020, 88, 113–119.
- Li, L.; Shi, L.; Jia, J.; Jiao, Y.; Gao, Y.; Liu, Y.; Dong, C.; Shuang, S. “On-off-on” detection of Fe3+ and F−, biological imaging, and its logic gate operation based on excitation-independent blue-fluorescent carbon dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117716.
- Cheng, Z.; Dong, H.; Liang, J.; Zhang, F.; Chen, X.; Du, L.; Tan, K. Highly selective fluorescent visual detection of perfluorooctane sulfonate via blue fluorescent carbon dots and berberine chloride hydrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 207, 262–269.
- Xu, X.; Mao, X.; Zhuang, J.; Lei, B.; Li, Y.; Li, W.; Liu, Y. PVA-coated fluorescent carbon dot nanocapsules as an optical amplifier for enhanced photosynthesis of lettuce. ACS Sustain. Chem. Eng. 2020, 8, 3938–3949.
- Chandra, S.; Pradhan, S.; Mitra, S.; Patra, P.; Bhattacharya, A.; Pramanik, P.; Goswami, A. High throughput electron transfer from carbon dots to chloroplast: A rationale of enhanced photosynthesis. Nanoscale 2014, 6, 3647–3655.
- Li, W.; Wu, S.; Zhang, H.; Zhang, X.; Zhuang, J.; Hu, C.; Wang, X. Enhanced Biological Photosynthetic Efficiency Using Light-Harvesting Engineering with Dual-Emissive Carbon Dots. Adv. Funct. Mater. 2018, 28, 1804004.
- Li, Y.; Pan, X.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Liu, Y. Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect. J. Hazard. Mater. 2021, 410, 124534.
- Guner, T.; Yuce, H.; Tascioglu, D.; Simsek, E.; Savaci, U.; Genc, A.; Turan, S.; Demir, M.M. Optimization and performance of nitrogen-doped carbon dots as a color conversion layer for white-LED applications. Beilstein J. Nanotechnol. 2019, 10, 2004–2013.
- Zhang, X.; Sun, Z.; Zhu, Z.; Luo, J.; Wu, Z.; Wang, Z. High-efficient, spherical and thermal-stable carbon silica fluorescent composite as rare earth-free phosphors for white LED. Ceram. Int. 2020, 46, 14706–14712.
- Cao, M.; Xia, C.; Xia, J.; Jiang, D.; Yu, C.; Li, H. A yellow carbon dots-based phosphor with high efficiency for white light-emitting devices. J. Lumin. 2019, 206, 97–104.
- Barman, B.K.; Nagao, T.; Nanda, K.K. Dual roles of a transparent polymer film containing dispersed N-doped carbon dots: A high-efficiency blue light converter and UV screen. Appl. Surf. Sci. 2020, 510, 145405.
- Ma, Q.; Zhang, Y.; Wu, G.; Yang, Q.; Yuan, Y.; Cheng, R.; Fang, H. Photovoltaic/spectrum performance analysis of a multifunctional solid spectral splitting covering for passive solar greenhouse roof. Energy Convers. Manag. 2022, 251, 114955.
- Shah, S.A.A.; Sayyad, M.H.; Sun, J.; Guo, Z. Recent advances and emerging trends of rare-earth-ion doped spectral conversion nanomaterials in perovskite solar cells. J. Rare Earth. 2021, in press.
- Cheng, Z.; Chai, R.; Ma, P.; Dai, Y.; Kang, X.; Lian, H.; Hou, Z.; Li, C.; Lin, J. Multiwalled carbon nanotubes and NaYF4:Yb3+/Er3+ nanoparticle-doped bilayer hydrogel for concurrent NIR-triggered drug release and up-conversion luminescence tagging. Langmuir 2013, 29, 9573–9580.
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086.
- Xu, X.; Li, W.; Hu, C.; Lei, B.; Zhang, X.; Li, Y.; Zhuang, J. Promoting the growth of mung bean plants through uptake and light conversion of NaYF4: Yb, CDs nanocomposites. ACS Sustain. Chem. Eng. 2020, 8, 9751–9762.
- Garfield, D.J.; Borys, N.J.; Hamed, S.M.; Torquato, N.A.; Tajon, C.A.; Tian, B.; Shevitski, B.; Barnard, E.S.; Suh, Y.D.; Aloni, S.; et al. Enrichment of molecular antenna triplets amplifies upconverting nanoparticle emission. Nat. Photonics 2018, 12, 402–407.
- Miyako, E.; Russier, J.; Mauro, M.; Cebrian, C.; Yawo, H.; Ménard-Moyon, C.; Hutchison, J.A.; Yudasaka, M.; Iijima, S.; De Cola, L.; et al. Photofunctional nanomodulators for bioexcitation. Angew. Chem. 2014, 126, 13337–13341.
- Nakatsuji, H.; Numata, T.; Morone, N.; Kaneko, S.; Mori, Y.; Imahori, H.; Murakami, T. Thermosensitive Ion Channel Activation in Single Neuronal Cells by Using Surface-Engineered Plasmonic Nanoparticles. Angew. Chem. Int Ed. Engl. 2015, 54, 11725–11729.
More
