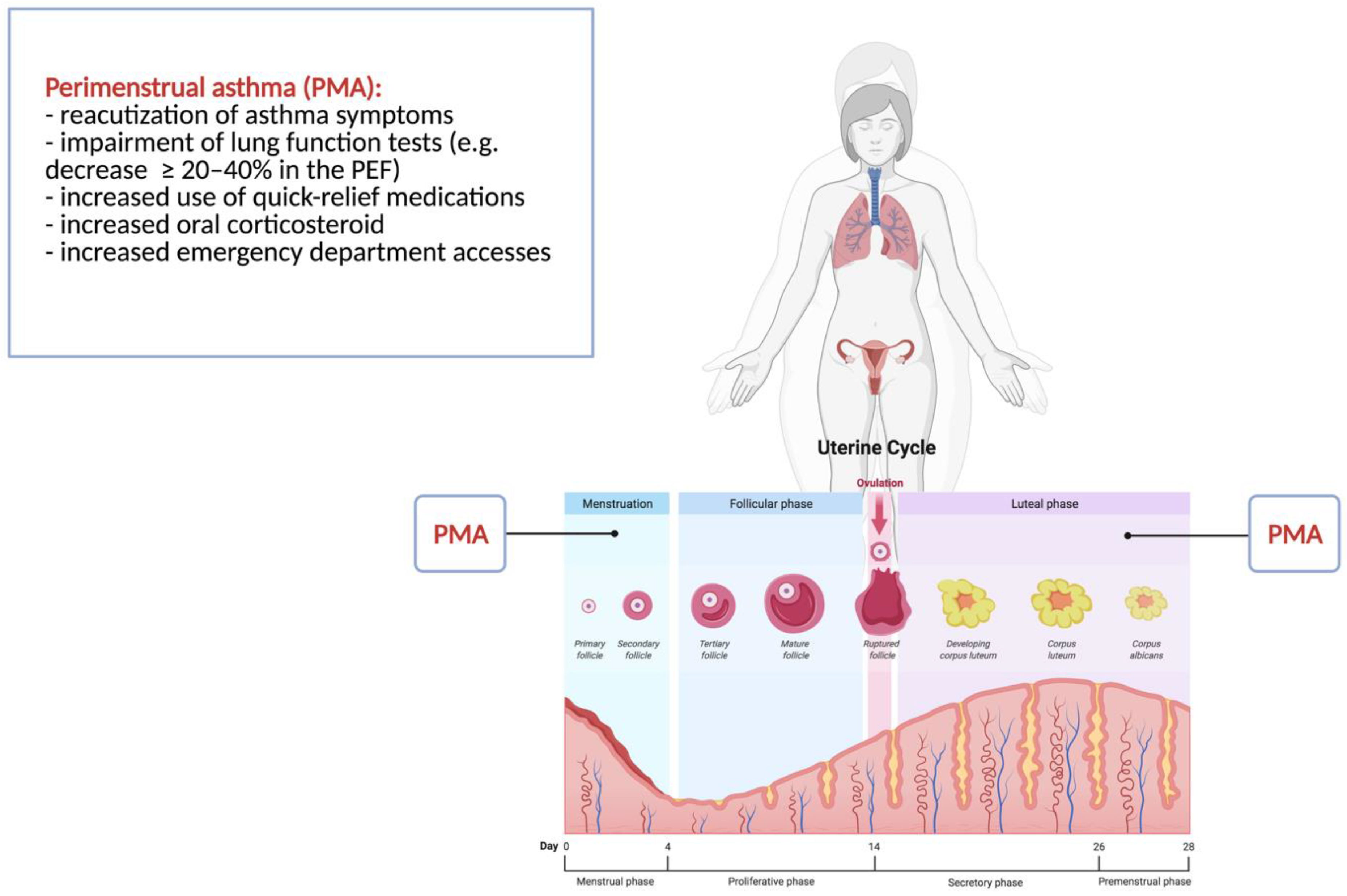
Asthma is a frequent medical condition in adolescence. The worsening of the most common symptoms perimenstrually is defined as perimenstrual asthma (PMA). The cause of PMA remains unclear, but a role for hormonal milieu is plausible. Data on PMA in adolescents are limited, and its management is not fully established. We aimed to discuss the PMA phenomenon in young females from pathophysiology to preventive strategies, focusing on the relationship with the hormonal pattern. The fluctuation of estrogens at ovulation and before menstruation and the progesterone secretion during the luteal phase and its subsequent withdrawal seem to be the culprits, because the deterioration of asthma is cyclical during the luteal phase and/or during the first days of the menstrual cycle.
- perimenstrual asthma
- sex hormones
- adolescents
- menstrual cycle
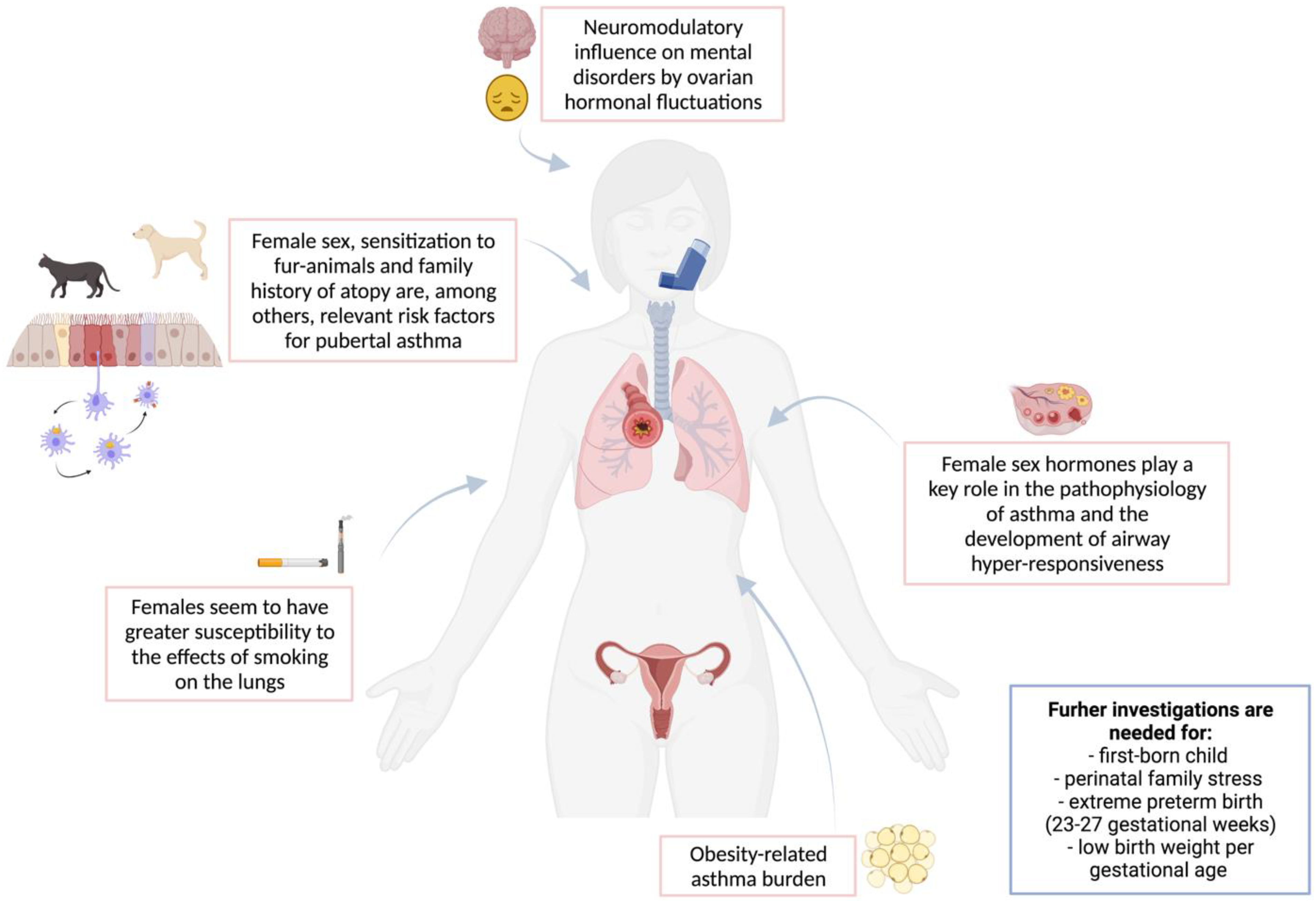
1. Asthma in Young Female Adolescents
2. Perimenstrual Asthma
3. Perimenstrual Asthma and Sex Hormones
References
- de Benedictis, D.; Bush, A. Asthma in Adolescence: Is There Any News?: Asthma in Adolescence. Pediatr. Pulmonol. 2017, 52, 129–138.
- Gore, F.M.; Bloem, P.J.N.; Patton, G.C.; Ferguson, J.; Joseph, V.; Coffey, C.; Sawyer, S.M.; Mathers, C.D. Global Burden of Disease in Young People Aged 10–24 Years: A Systematic Analysis. Lancet 2011, 377, 2093–2102.
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800.
- Leynaert, B.; Sunyer, J.; Garcia-Esteban, R.; Svanes, C.; Jarvis, D.; Cerveri, I.; Dratva, J.; Gislason, T.; Heinrich, J.; Janson, C.; et al. Gender Differences in Prevalence, Diagnosis and Incidence of Allergic and Non-Allergic Asthma: A Population-Based Cohort. Thorax 2012, 67, 625–631.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention; GINA: London, UK, 2021.
- Chipps, B.E.; Bacharier, L.B.; Farrar, J.R.; Jackson, D.J.; Murphy, K.R.; Phipatanakul, W.; Szefler, S.J.; Teague, W.G.; Zeiger, R.S. The Pediatric Asthma Yardstick: Practical Recommendations for a Sustained Step-up in Asthma Therapy for Children with Inadequately Controlled Asthma. Ann. Allergy Asthma Immunol. 2018, 120, 559–579.e11.
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic Endotypes and Phenotypes of Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440.
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. Asthma Endotyping and Biomarkers in Childhood Asthma. Pediatr. Allergy Immunol. Pulmonol. 2018, 31, 44–55.
- Di Cicco, M.; D’Elios, S.; Peroni, D.G.; Comberiati, P. The Role of Atopy in Asthma Development and Persistence. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 131–137.
- Calcaterra, V.; Verduci, E.; Ghezzi, M.; Cena, H.; Pascuzzi, M.C.; Regalbuto, C.; Lamberti, R.; Rossi, V.; Manuelli, M.; Bosetti, A.; et al. Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients 2021, 13, 3708.
- Beuther, D.A.; Sutherland, E.R. Overweight, Obesity, and Incident Asthma: A Meta-Analysis of Prospective Epidemiologic Studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666.
- Thomsen, S.F.; Ulrik, C.S.; Kyvik, K.O.; Sørensen, T.I.A.; Posthuma, D.; Skadhauge, L.R.; Steffensen, I.; Backer, V. Association between Obesity and Asthma in a Twin Cohort. Allergy 2007, 62, 1199–1204.
- Cibella, F.; Cuttitta, G.; La Grutta, S.; Melis, M.R.; Bucchieri, S.; Viegi, G. A Cross-Sectional Study Assessing the Relationship between BMI, Asthma, Atopy, and ENO among Schoolchildren. Ann. Allergy Asthma Immunol. 2011, 107, 330–336.
- Quinto, K.B.; Zuraw, B.L.; Poon, K.-Y.T.; Chen, W.; Schatz, M.; Christiansen, S.C. The Association of Obesity and Asthma Severity and Control in Children. J. Allergy Clin. Immunol. 2011, 128, 964–969.
- Vijayakanthi, N.; Greally, J.M.; Rastogi, D. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics 2016, 137, e20150812.
- Chen, Y.C.; Dong, G.H.; Lin, K.C.; Lee, Y.L. Gender Difference of Childhood Overweight and Obesity in Predicting the Risk of Incident Asthma: A Systematic Review and Meta-Analysis. Obes. Rev. 2013, 14, 222–231.
- Forno, E.; Lescher, R.; Strunk, R.; Weiss, S.; Fuhlbrigge, A.; Celedón, J.C. Childhood Asthma Management Program Research Group Decreased Response to Inhaled Steroids in Overweight and Obese Asthmatic Children. J. Allergy Clin. Immunol. 2011, 127, 741–749.
- Belamarich, P.F.; Luder, E.; Kattan, M.; Mitchell, H.; Islam, S.; Lynn, H.; Crain, E.F. Do Obese Inner-City Children with Asthma Have More Symptoms than Nonobese Children with Asthma? Pediatrics 2000, 106, 1436–1441.
- Kattan, M.; Kumar, R.; Bloomberg, G.R.; Mitchell, H.E.; Calatroni, A.; Gergen, P.J.; Kercsmar, C.M.; Visness, C.M.; Matsui, E.C.; Steinbach, S.F.; et al. Asthma Control, Adiposity, and Adipokines among Inner-City Adolescents. J. Allergy Clin. Immunol. 2010, 125, 584–592.
- Messiah, S.E.; Arheart, K.L.; Lipshultz, S.E.; Miller, T.L. Ethnic Group Differences in Waist Circumference Percentiles among U.S. Children and Adolescents: Estimates from the 1999–2008 National Health and Nutrition Examination Surveys. Metab. Syndr. Relat. Disord. 2011, 9, 297–303.
- Huang, S.L.; Shiao, G.; Chou, P. Association between Body Mass Index and Allergy in Teenage Girls in Taiwan. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1999, 29, 323–329.
- Schachter, L.M.; Peat, J.K.; Salome, C.M. Asthma and Atopy in Overweight Children. Thorax 2003, 58, 1031–1035.
- Jensen, M.E.; Gibson, P.G.; Collins, C.E.; Wood, L.G. Airway and Systemic Inflammation in Obese Children with Asthma. Eur. Respir. J. 2013, 42, 1012–1019.
- Ali, Z.; Ulrik, C.S. Obesity and Asthma: A Coincidence or a Causal Relationship? A Systematic Review. Respir. Med. 2013, 107, 1287–1300.
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Jarjour, N.N.; et al. Obesity and Asthma: An Association Modified by Age of Asthma Onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493.e2.
- Couriel, J. Asthma in Adolescence. Paediatr. Respir. Rev. 2003, 4, 47–54.
- Kaur, B.; Anderson, H.R.; Austin, J.; Burr, M.; Harkins, L.S.; Strachan, D.P.; Warner, J.O. Prevalence of Asthma Symptoms, Diagnosis, and Treatment in 12–14 Year Old Children across Great Britain (International Study of Asthma and Allergies in Childhood, ISAAC UK). BMJ 1998, 316, 118–124.
- Siersted, H.C.; Boldsen, J.; Hansen, H.S.; Mostgaard, G.; Hyldebrandt, N. Population Based Study of Risk Factors for Underdiagnosis of Asthma in Adolescence: Odense Schoolchild Study. BMJ 1998, 316, 651–655; discussion 655–656.
- Burgess, J.A.; Matheson, M.C.; Gurrin, L.C.; Byrnes, G.B.; Adams, K.S.; Wharton, C.L.; Giles, G.G.; Jenkins, M.A.; Hopper, J.L.; Abramson, M.J.; et al. Factors Influencing Asthma Remission: A Longitudinal Study from Childhood to Middle Age. Thorax 2011, 66, 508–513.
- Andersson, M.; Hedman, L.; Bjerg, A.; Forsberg, B.; Lundbäck, B.; Rönmark, E. Remission and Persistence of Asthma Followed from 7 to 19 Years of Age. Pediatrics 2013, 132, e435–e442.
- Roorda, R.J.; Gerritsen, J.; van Aalderen, W.M.; Schouten, J.P.; Veltman, J.C.; Weiss, S.T.; Knol, K. Follow-up of Asthma from Childhood to Adulthood: Influence of Potential Childhood Risk Factors on the Outcome of Pulmonary Function and Bronchial Responsiveness in Adulthood. J. Allergy Clin. Immunol. 1994, 93, 575–584.
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A Longitudinal, Population-Based, Cohort Study of Childhood Asthma Followed to Adulthood. N. Engl. J. Med. 2003, 349, 1414–1422.
- Phelan, P.D.; Robertson, C.F.; Olinsky, A. The Melbourne Asthma Study: 1964–1999. J. Allergy Clin. Immunol. 2002, 109, 189–194.
- Sekerel, B.E.; Civelek, E.; Karabulut, E.; Yildirim, S.; Tuncer, A.; Adalioglu, G. Are Risk Factors of Childhood Asthma Predicting Disease Persistence in Early Adulthood Different in the Developing World? Allergy 2006, 61, 869–877.
- Vink, N.M.; Postma, D.S.; Schouten, J.P.; Rosmalen, J.G.M.; Boezen, H.M. Gender Differences in Asthma Development and Remission during Transition through Puberty: The TRacking Adolescents’ Individual Lives Survey (TRAILS) Study. J. Allergy Clin. Immunol. 2010, 126, 498–504.e1–6.
- Anderson, H.R.; Pottier, A.C.; Strachan, D.P. Asthma from Birth to Age 23: Incidence and Relation to Prior and Concurrent Atopic Disease. Thorax 1992, 47, 537–542.
- Hovland, V.; Riiser, A.; Mowinckel, P.; Carlsen, K.-H.; Lødrup Carlsen, K.C. Early Risk Factors for Pubertal Asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 164–176.
- Andersson, M.; Bjerg, A.; Forsberg, B.; Lundbäck, B.; Rönmark, E. The Clinical Expression of Asthma in Schoolchildren Has Changed between 1996 and 2006. Pediatr. Allergy Immunol. 2010, 21, 859–866.
- Crump, C.; Winkleby, M.A.; Sundquist, J.; Sundquist, K. Risk of Asthma in Young Adults Who Were Born Preterm: A Swedish National Cohort Study. Pediatrics 2011, 127, e913–e920.
- Johnson, C.C.; Peterson, E.L.; Joseph, C.L.M.; Ownby, D.R.; Breslau, N. Birth Weight and Asthma Incidence by Asthma Phenotype Pattern in a Racially Diverse Cohort Followed through Adolescence. J. Asthma 2015, 52, 1006–1012.
- McCleary, N.; Nwaru, B.I.; Nurmatov, U.B.; Critchley, H.; Sheikh, A. Endogenous and Exogenous Sex Steroid Hormones in Asthma and Allergy in Females: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. 2018, 141, 1510–1513.e8.
- Salam, M.T.; Wenten, M.; Gilliland, F.D. Endogenous and Exogenous Sex Steroid Hormones and Asthma and Wheeze in Young Women. J. Allergy Clin. Immunol. 2006, 117, 1001–1007.
- Bjerg-Bäcklund, A.; Bäcklund, A.B.; Perzanowski, M.S.; Platts-Mills, T.; Sandström, T.; Lundbäck, B.; Rönmark, E. Asthma during the Primary School Ages--Prevalence, Remission and the Impact of Allergic Sensitization. Allergy 2006, 61, 549–555.
- Vonk, J.M.; Boezen, H.M. Predicting Adult Asthma in Childhood. Curr. Opin. Pulm. Med. 2006, 12, 42–47.
- Rönmark, E.; Lindberg, A.; Watson, L.; Lundbäck, B. Outcome and Severity of Adult Onset Asthma--Report from the Obstructive Lung Disease in Northern Sweden Studies (OLIN). Respir. Med. 2007, 101, 2370–2377.
- Arshad, S.H.; Raza, A.; Lau, L.; Bawakid, K.; Karmaus, W.; Zhang, H.; Ewart, S.; Patil, V.; Roberts, G.; Kurukulaaratchy, R. Pathophysiological Characterization of Asthma Transitions across Adolescence. Respir. Res. 2014, 15, 153.
- Goksör, E.; Åmark, M.; Alm, B.; Ekerljung, L.; Lundbäck, B.; Wennergren, G. High Risk of Adult Asthma Following Severe Wheezing in Early Life. Pediatr. Pulmonol. 2015, 50, 789–797.
- Almqvist, C.; Worm, M.; Leynaert, B.; Working group of GA2LEN WP 2.5 Gender. Impact of Gender on Asthma in Childhood and Adolescence: A GA2LEN Review. Allergy 2008, 63, 47–57.
- Meyer, M.R.; Haas, E.; Barton, M. Gender Differences of Cardiovascular Disease: New Perspectives for Estrogen Receptor Signaling. Hypertens. Dallas Tex 2006, 47, 1019–1026.
- Most, W.; van der Wee-Pals, L.; Ederveen, A.; Papapoulos, S.; Löwik, C. Ovariectomy and Orchidectomy Induce a Transient Increase in the Osteoclastogenic Potential of Bone Marrow Cells in the Mouse. Bone 1997, 20, 27–30.
- Gillies, G.E.; Murray, H.E.; Dexter, D.; McArthur, S. Sex Dimorphisms in the Neuroprotective Effects of Estrogen in an Animal Model of Parkinson’s Disease. Pharmacol. Biochem. Behav. 2004, 78, 513–522.
- Matsubara, S.; Swasey, C.H.; Loader, J.E.; Dakhama, A.; Joetham, A.; Ohnishi, H.; Balhorn, A.; Miyahara, N.; Takeda, K.; Gelfand, E.W. Estrogen Determines Sex Differences in Airway Responsiveness after Allergen Exposure. Am. J. Respir. Cell Mol. Biol. 2008, 38, 501–508.
- Dimitropoulou, C.; White, R.E.; Ownby, D.R.; Catravas, J.D. Estrogen Reduces Carbachol-Induced Constriction of Asthmatic Airways by Stimulating Large-Conductance Voltage and Calcium-Dependent Potassium Channels. Am. J. Respir. Cell Mol. Biol. 2005, 32, 239–247.
- Degano, B.; Prévost, M.C.; Berger, P.; Molimard, M.; Pontier, S.; Rami, J.; Escamilla, R. Estradiol Decreases the Acetylcholine-Elicited Airway Reactivity in Ovariectomized Rats through an Increase in Epithelial Acetylcholinesterase Activity. Am. J. Respir. Crit. Care Med. 2001, 164, 1849–1854.
- Melgert, B.N.; Ray, A.; Hylkema, M.N.; Timens, W.; Postma, D.S. Are There Reasons Why Adult Asthma Is More Common in Females? Curr. Allergy Asthma Rep. 2007, 7, 143–150.
- Corteling, R.; Trifilieff, A. Gender Comparison in a Murine Model of Allergen-Driven Airway Inflammation and the Response to Budesonide Treatment. BMC Pharmacol. 2004, 4, 4.
- Riffo-Vasquez, Y.; Ligeiro de Oliveira, A.P.; Page, C.P.; Spina, D.; Tavares-de-Lima, W. Role of Sex Hormones in Allergic Inflammation in Mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2007, 37, 459–470.
- Carey, M.A.; Card, J.W.; Bradbury, J.A.; Moorman, M.P.; Haykal-Coates, N.; Gavett, S.H.; Graves, J.P.; Walker, V.R.; Flake, G.P.; Voltz, J.W.; et al. Spontaneous Airway Hyperresponsiveness in Estrogen Receptor-Alpha-Deficient Mice. Am. J. Respir. Crit. Care Med. 2007, 175, 126–135.
- Taube, C.; Wei, X.; Swasey, C.H.; Joetham, A.; Zarini, S.; Lively, T.; Takeda, K.; Loader, J.; Miyahara, N.; Kodama, T.; et al. Mast Cells, Fc Epsilon RI, and IL-13 Are Required for Development of Airway Hyperresponsiveness after Aerosolized Allergen Exposure in the Absence of Adjuvant. J. Immunol. Baltim. Md 2004, 172, 6398–6406.
- Melgert, B.N.; Postma, D.S.; Kuipers, I.; Geerlings, M.; Luinge, M.A.; van der Strate, B.W.A.; Kerstjens, H.A.M.; Timens, W.; Hylkema, M.N. Female Mice Are More Susceptible to the Development of Allergic Airway Inflammation than Male Mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2005, 35, 1496–1503.
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal Involution by Age-Associated Oxidative Stress and Its Acceleration by Loss of Sex Steroids. J. Biol. Chem. 2007, 282, 27285–27297.
- Almeida, M.; Han, L.; Ambrogini, E.; Bartell, S.M.; Manolagas, S.C. Oxidative Stress Stimulates Apoptosis and Activates NF-ΚB in Osteoblastic Cells via a PKCβ/P66shc Signaling Cascade: Counter Regulation by Estrogens or Androgens. Mol. Endocrinol. 2010, 24, 2030–2037.
- Olsen, N.J.; Kovacs, W.J. Gonadal Steroids and Immunity. Endocr. Rev. 1996, 17, 369–384.
- Talal, N. Sex Steroid Hormones and Systemic Lupus Erythematosus. Arthritis Rheum. 1981, 24, 1054–1056.
- Bijlsma, J.W.J.; Van Den Brink, H.R. Estrogens and Rheumatoid Arthritis. Am. J. Reprod. Immunol. 1992, 28, 231–234.
- Cutolo, M.; Wilder, R.L. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum. Dis. Clin. N. Am. 2000, 26, 825–839.
- Lang, T.J. Estrogen as an Immunomodulator. Clin. Immunol. 2004, 113, 224–230.
- Carruba, G.; D’Agostino, P.; Miele, M.; Calabrò, M.; Barbera, C.; Bella, G.D.; Milano, S.; Ferlazzo, V.; Caruso, R.; Rosa, M.L.; et al. Estrogen Regulates Cytokine Production and Apoptosis in PMA-Differentiated, Macrophage-like U937 Cells: Estrogen Regulates Cytokine Production in U937 Cells. J. Cell. Biochem. 2003, 90, 187–196.
- Mor, G.; Sapi, E.; Abrahams, V.M.; Rutherford, T.; Song, J.; Hao, X.-Y.; Muzaffar, S.; Kohen, F. Interaction of the Estrogen Receptors with the Fas Ligand Promoter in Human Monocytes. J. Immunol. 2003, 170, 114–122.
- Skobeloff, E.M.; Spivey, W.H.; St Clair, S.S.; Schoffstall, J.M. The Influence of Age and Sex on Asthma Admissions. JAMA 1992, 268, 3437–3440.
- Hyndman, S.J.; Williams, D.R.; Merrill, S.L.; Lipscombe, J.M.; Palmer, C.R. Rates of Admission to Hospital for Asthma. BMJ 1994, 308, 1596–1600.
- Bloomberg, G.R.; Trinkaus, K.M.; Fisher, E.B.; Musick, J.R.; Strunk, R.C. Hospital Readmissions for Childhood Asthma: A 10-Year Metropolitan Study. Am. J. Respir. Crit. Care Med. 2003, 167, 1068–1076.
- Postma, D.S. Gender Differences in Asthma Development and Progression. Gend. Med. 2007, 4 (Suppl. B), S133–S146.
- Dratva, J.; Schindler, C.; Curjuric, I.; Stolz, D.; Macsali, F.; Gomez, F.R.; Zemp, E. SAPALDIA Team Perimenstrual Increase in Bronchial Hyperreactivity in Premenopausal Women: Results from the Population-Based SAPALDIA 2 Cohort. J. Allergy Clin. Immunol. 2010, 125, 823–829.
- Liptzin, D.R.; Landau, L.I.; Taussig, L.M. Sex and the Lung: Observations, Hypotheses, and Future Directions. Pediatr. Pulmonol. 2015, 50, 1159–1169.
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr. Rev. 2012, 33, 1–47.
- Gibbs, C.J.; Coutts, I.I.; Lock, R.; Finnegan, O.C.; White, R.J. Premenstrual Exacerbation of Asthma. Thorax 1984, 39, 833–836.
- Pauli, B.D.; Reid, R.L.; Munt, P.W.; Wigle, R.D.; Forkert, L. Influence of the Menstrual Cycle on Airway Function in Asthmatic and Normal Subjects. Am. Rev. Respir. Dis. 1989, 140, 358–362.
- Ensom, M.H.H.; Chong, G.; Beaudin, B.; Bai, T.R. Estradiol in Severe Asthma with Premenstrual Worsening. Ann. Pharmacother. 2003, 37, 1610–1613.
- Ensom, M.H.; Chong, E.; Carter, D. Premenstrual Symptoms in Women with Premenstrual Asthma. Pharmacotherapy 1999, 19, 374–382.
- Oguzulgen, I.K.; Turktas, H.; Erbas, D. Airway Inflammation in Premenstrual Asthma. J. Asthma 2002, 39, 517–522.
- Jung, W.J.; Lee, S.Y.; Choi, S.I.; Kim, B.-K.; Lee, E.J.; Choi, J. Population-Based Study of the Association between Asthma and Exogenous Female Sex Hormone Use. BMJ Open 2021, 11, e046400.
- Bernstein, J.A. Progestogen Sensitization: A Unique Female Presentation of Anaphylaxis. Curr. Allergy Asthma Rep. 2020, 20, 4.
- Pedersen, S.E.; Bateman, E.D.; Bousquet, J.; Busse, W.W.; Yoxall, S.; Clark, T.J. Gaining Optimal Asthma controL Steering Committee and Investigators Determinants of Response to Fluticasone Propionate and Salmeterol/Fluticasone Propionate Combination in the Gaining Optimal Asthma ControL Study. J. Allergy Clin. Immunol. 2007, 120, 1036–1042.
- Tse, S.M.; Coull, B.A.; Sordillo, J.E.; Datta, S.; Gold, D.R. Gender- and Age-Specific Risk Factors for Wheeze from Birth through Adolescence. Pediatr. Pulmonol. 2015, 50, 955–962.
- Rosewich, M.; Schulze, J.; Eickmeier, O.; Adler, S.; Rose, M.A.; Schubert, R.; Zielen, S. Early Impact of Smoking on Lung Function, Health, and Well-Being in Adolescents. Pediatr. Pulmonol. 2012, 47, 692–699.
- Yoo, S.; Kim, H.B.; Lee, S.Y.; Kim, B.S.; Kim, J.H.; Yu, J.; Kim, B.J.; Lee, D.H.; Seong, M.W.; Hong, S.J. Effect of Active Smoking on Asthma Symptoms, Pulmonary Function, and BHR in Adolescents. Pediatr. Pulmonol. 2009, 44, 954–961.
- Silvestri, M.; Franchi, S.; Pistorio, A.; Petecchia, L.; Rusconi, F. Smoke Exposure, Wheezing, and Asthma Development: A Systematic Review and Meta-Analysis in Unselected Birth Cohorts. Pediatr. Pulmonol. 2015, 50, 353–362.
- Hedman, L.; Bjerg, A.; Sundberg, S.; Forsberg, B.; Rönmark, E. Both Environmental Tobacco Smoke and Personal Smoking Is Related to Asthma and Wheeze in Teenagers. Thorax 2011, 66, 20–25.
- Thacher, J.D.; Gruzieva, O.; Pershagen, G.; Neuman, Å.; Wickman, M.; Kull, I.; Melén, E.; Bergström, A. Pre- and Postnatal Exposure to Parental Smoking and Allergic Disease through Adolescence. Pediatrics 2014, 134, 428–434.
- Guerra, S.; Stern, D.A.; Zhou, M.; Sherrill, D.L.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Combined Effects of Parental and Active Smoking on Early Lung Function Deficits: A Prospective Study from Birth to Age 26 Years. Thorax 2013, 68, 1021–1028.
- Chalmers, G.W.; Macleod, K.J.; Little, S.A.; Thomson, L.J.; McSharry, C.P.; Thomson, N.C. Influence of Cigarette Smoking on Inhaled Corticosteroid Treatment in Mild Asthma. Thorax 2002, 57, 226–230.
- Cooke, A.; Fergeson, J.; Bulkhi, A.; Casale, T.B. The Electronic Cigarette: The Good, the Bad, and the Ugly. J. Allergy Clin. Immunol. Pract. 2015, 3, 498–505.
- Polosa, R.; Morjaria, J.; Caponnetto, P.; Caruso, M.; Strano, S.; Battaglia, E.; Russo, C. Effect of Smoking Abstinence and Reduction in Asthmatic Smokers Switching to Electronic Cigarettes: Evidence for Harm Reversal. Int. J. Environ. Res. Public. Health 2014, 11, 4965–4977.
- Giroud, C.; de Cesare, M.; Berthet, A.; Varlet, V.; Concha-Lozano, N.; Favrat, B. E-Cigarettes: A Review of New Trends in Cannabis Use. Int. J. Environ. Res. Public. Health 2015, 12, 9988–10008.
- Randolph, C.C.; Fraser, B. Stressors and Concerns in Teen Asthma. Allergy Asthma Proc. 1998, 19, 193–203.
- Bender, B.; Zhang, L. Negative Affect, Medication Adherence, and Asthma Control in Children. J. Allergy Clin. Immunol. 2008, 122, 490–495.
- Richardson, L.P.; Lozano, P.; Russo, J.; McCauley, E.; Bush, T.; Katon, W. Asthma Symptom Burden: Relationship to Asthma Severity and Anxiety and Depression Symptoms. Pediatrics 2006, 118, 1042–1051.
- Vila, G.; Nollet-Clémençon, C.; de Blic, J.; Falissard, B.; Mouren-Simeoni, M.C.; Scheinmann, P. Assessment of Anxiety Disorders in Asthmatic Children. Psychosomatics 1999, 40, 404–413.
- Burkhart, P.V.; Rayens, M.K. Self-Concept and Health Locus of Control: Factors Related to Children’s Adherence to Recommended Asthma Regimen. Pediatr. Nurs. 2005, 31, 404–409.
- Bruzzese, J.-M.; Fisher, P.H.; Lemp, N.; Warner, C.M. Asthma and Social Anxiety in Adolescents. J. Pediatr. 2009, 155, 398–403.
- Vila, G.; Nollet-Clemençon, C.; de Blic, J.; Mouren-Simeoni, M.C.; Scheinmann, P. Prevalence of DSM IV Anxiety and Affective Disorders in a Pediatric Population of Asthmatic Children and Adolescents. J. Affect. Disord. 2000, 58, 223–231.
- Buhrmester, D.; Furman, W. The Development of Companionship and Intimacy. Child Dev. 1987, 58, 1101–1113.
- Kyngäs, H. Support Network of Adolescents with Chronic Disease: Adolescents’ Perspective. Nurs. Health Sci. 2004, 6, 287–293.
- KyngAs, H.A.; Kroll, T.; Duffy, M.E. Compliance in Adolescents with Chronic Diseases: A Review. J. Adolesc. Health 2000, 26, 379–388.
- Price, J.F. Issues in Adolescent Asthma: What Are the Needs? Thorax 1996, 51 (Suppl. 1), S13–S17.
- Fitzgerald, D. Non-Compliance in Adolescents with Chronic Lung Disease: Causative Factors and Practical Approach. Paediatr. Respir. Rev. 2001, 2, 260–267.
- McLean, C.P.; Anderson, E.R. Brave Men and Timid Women? A Review of the Gender Differences in Fear and Anxiety. Clin. Psychol. Rev. 2009, 29, 496–505.
- Breier, A.; Charney, D.S.; Heninger, G.R. Agoraphobia with Panic Attacks. Development, Diagnostic Stability, and Course of Illness. Arch. Gen. Psychiatry 1986, 43, 1029–1036.
- Freeman, E.W. Premenstrual Syndrome and Premenstrual Dysphoric Disorder: Definitions and Diagnosis. Psychoneuroendocrinology 2003, 28 (Suppl. 3), 25–37.
- Gonda, X.; Telek, T.; Juhász, G.; Lazary, J.; Vargha, A.; Bagdy, G. Patterns of Mood Changes throughout the Reproductive Cycle in Healthy Women without Premenstrual Dysphoric Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1782–1788.
- Kaspi, S.P.; Otto, M.W.; Pollack, M.H.; Eppinger, S.; Rosenbaum, J.F. Premenstrual Exacerbation of Symptoms in Women with Panic Disorder. J. Anxiety Disord. 1994, 8, 131–138.
- Kornstein, S.G.; Harvey, A.T.; Rush, A.J.; Wisniewski, S.R.; Trivedi, M.H.; Svikis, D.S.; McKenzie, N.D.; Bryan, C.; Harley, R. Self-Reported Premenstrual Exacerbation of Depressive Symptoms in Patients Seeking Treatment for Major Depression. Psychol. Med. 2005, 35, 683–692.
- Rees, L. An Aetiological Study of Premenstrual Asthma. J. Psychosom. Res. 1963, 7, 191–197.
- Agarwal, A.K.; Shah, A. Menstrual-Linked Asthma. J. Asthma 1997, 34, 539–545.
- Vrieze, A.; Postma, D.S.; Kerstjens, H.A.M. Perimenstrual Asthma: A Syndrome without Known Cause or Cure. J. Allergy Clin. Immunol. 2003, 112, 271–282.
- Graziottin, A.; Serafini, A. Perimenstrual Asthma: From Pathophysiology to Treatment Strategies. Multidiscip. Respir. Med. 2016, 11, 30.
- Rao, C.K.; Moore, C.G.; Bleecker, E.; Busse, W.W.; Calhoun, W.; Castro, M.; Chung, K.F.; Erzurum, S.C.; Israel, E.; Curran-Everett, D.; et al. Characteristics of Perimenstrual Asthma and Its Relation to Asthma Severity and Control: Data from the Severe Asthma Research Program. Chest 2013, 143, 984–992.
- Frank, R.T. The Hormonal Causes of Premenstrual Tension. Arch. Neurol. Psychiatry 1931, 26, 1053–1057.
- Chandler, M.H.; Schuldheisz, S.; Phillips, B.A.; Muse, K.N. Premenstrual Asthma: The Effect of Estrogen on Symptoms, Pulmonary Function, and Beta 2-Receptors. Pharmacotherapy 1997, 17, 224–234.
- Nakasato, H.; Ohrui, T.; Sekizawa, K.; Matsui, T.; Yamaya, M.; Tamura, G.; Sasaki, H. Prevention of Severe Premenstrual Asthma Attacks by Leukotriene Receptor Antagonist. J. Allergy Clin. Immunol. 1999, 104, 585–588.
- Murphy, V.E.; Gibson, P.G. Premenstrual Asthma: Prevalence, Cycle-to-Cycle Variability and Relationship to Oral Contraceptive Use and Menstrual Symptoms. J. Asthma 2008, 45, 696–704.
- Brenner, B.E.; Holmes, T.M.; Mazal, B.; Camargo, C.A. Relation between Phase of the Menstrual Cycle and Asthma Presentations in the Emergency Department. Thorax 2005, 60, 806–809.
- Becklake, M.R.; Kauffmann, F. Gender Differences in Airway Behaviour over the Human Life Span. Thorax 1999, 54, 1119–1138.
- DeBoer, M.D.; Phillips, B.R.; Mauger, D.T.; Zein, J.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.M.; Myers, R.; Ross, K.R.; Chmiel, J.; et al. Effects of Endogenous Sex Hormones on Lung Function and Symptom Control in Adolescents with Asthma. BMC Pulm. Med. 2018, 18, 58.
- Zein, J.G.; Erzurum, S.C. Asthma Is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 28.
- Heitkemper, M.M.; Cain, K.C.; Jarrett, M.E.; Burr, R.L.; Hertig, V.; Bond, E.F. Symptoms across the Menstrual Cycle in Women with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2003, 98, 420–430.
- Rubtsova, K.; Marrack, P.; Rubtsov, A.V. Sexual Dimorphism in Autoimmunity. J. Clin. Investig. 2015, 125, 2187–2193.
- Zandman-Goddard, G.; Peeva, E.; Shoenfeld, Y. Gender and Autoimmunity. Autoimmun. Rev. 2007, 6, 366–372.
- Wijga, A.; Tabak, C.; Postma, D.S.; Kerkhof, M.; Wieringa, M.H.; Hoekstra, M.O.; Brunekreef, B.; de Jongste, J.C.; Smit, H.A. Sex Differences in Asthma during the First 8 Years of Life: The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) Birth Cohort Study. J. Allergy Clin. Immunol. 2011, 127, 275–277.
- Sears, M.R.; Burrows, B.; Flannery, E.M.; Herbison, G.P.; Holdaway, M.D. Atopy in Childhood. I. Gender and Allergen Related Risks for Development of Hay Fever and Asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1993, 23, 941–948.
- Johnson, C.C.; Peterson, E.L.; Ownby, D.R. Gender Differences in Total and Allergen-Specific Immunoglobulin E (IgE) Concentrations in a Population-Based Cohort from Birth to Age Four Years. Am. J. Epidemiol. 1998, 147, 1145–1152.
- Kulig, M.; Tacke, U.; Forster, J.; Edenharter, G.; Bergmann, R.; Lau, S.; Wahn, V.; Zepp, F.; Wahn, U. Serum IgE Levels during the First 6 Years of Life. J. Pediatr. 1999, 134, 453–458.
- Mohammad, H.R.; Belgrave, D.; Kopec Harding, K.; Murray, C.S.; Simpson, A.; Custovic, A. Age, Sex and the Association between Skin Test Responses and IgE Titres with Asthma. Pediatr. Allergy Immunol. 2016, 27, 313–319.
- Uekert, S.J.; Akan, G.; Evans, M.D.; Li, Z.; Roberg, K.; Tisler, C.; Dasilva, D.; Anderson, E.; Gangnon, R.; Allen, D.B.; et al. Sex-Related Differences in Immune Development and the Expression of Atopy in Early Childhood. J. Allergy Clin. Immunol. 2006, 118, 1375–1381.
- Douin-Echinard, V.; Calippe, B.; Billon-Galès, A.; Fontaine, C.; Lenfant, F.; Trémollières, F.; Bayard, F.; Guéry, J.-C.; Arnal, J.-F.; Gourdy, P. Estradiol Administration Controls Eosinophilia through Estrogen Receptor-Alpha Activation during Acute Peritoneal Inflammation. J. Leukoc. Biol. 2011, 90, 145–154.
- Huygen, K.; Palfliet, K. Strain Variation in Interferon Gamma Production of BCG-Sensitized Mice Challenged with PPD II. Importance of One Major Autosomal Locus and Additional Sexual Influences. Cell. Immunol. 1984, 85, 75–81.
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential Estrogen Receptor Gene Expression in Human Peripheral Blood Mononuclear Cell Populations. Immunol. Lett. 2005, 97, 107–113.
- Björnström, L.; Sjöberg, M. Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Mol. Endocrinol. 2005, 19, 833–842.
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R.; Bland, K.I. Estrogen Action Via the G Protein-Coupled Receptor, GPR30: Stimulation of Adenylyl Cyclase and CAMP-Mediated Attenuation of the Epidermal Growth Factor Receptor-to-MAPK Signaling Axis. Mol. Endocrinol. 2002, 16, 70–84.
- Fuentes, N.; Silveyra, P. Endocrine Regulation of Lung Disease and Inflammation. Exp. Biol. Med. 2018, 243, 1313–1322.
- Massaro, D.; Massaro, G.D. Estrogen Regulates Pulmonary Alveolar Formation, Loss, and Regeneration in Mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L1154–L1159.
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574.
- Ambhore, N.S.; Kalidhindi, R.S.R.; Loganathan, J.; Sathish, V. Role of Differential Estrogen Receptor Activation in Airway Hyperreactivity and Remodeling in a Murine Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2019, 61, 469–480.
- Itoga, M.; Konno, Y.; Moritoki, Y.; Saito, Y.; Ito, W.; Tamaki, M.; Kobayashi, Y.; Kayaba, H.; Kikuchi, Y.; Chihara, J.; et al. Correction: G-Protein-Coupled Estrogen Receptor Agonist Suppresses Airway Inflammation in a Mouse Model of Asthma through IL-10. PLoS ONE 2015, 10, e0136326.
- Aravamudan, B.; Goorhouse, K.J.; Unnikrishnan, G.; Thompson, M.A.; Pabelick, C.M.; Hawse, J.R.; Prakash, Y.S.; Sathish, V. Differential Expression of Estrogen Receptor Variants in Response to Inflammation Signals in Human Airway Smooth Muscle: Expression of estrogen receptor variants in the airway. J. Cell. Physiol. 2017, 232, 1754–1760.
- Ambhore, N.S.; Katragadda, R.; Raju Kalidhindi, R.S.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Estrogen Receptor Beta Signaling Inhibits PDGF Induced Human Airway Smooth Muscle Proliferation. Mol. Cell. Endocrinol. 2018, 476, 37–47.
- Hocking, D.C. Fibronectin Matrix Deposition and Cell Contractility. Chest 2002, 122, 275S–278S.
- Fahy, J.V. Type 2 Inflammation in Asthma--Present in Most, Absent in Many. Nat. Rev. Immunol. 2015, 15, 57–65.
- Ray, A.; Raundhal, M.; Oriss, T.B.; Ray, P.; Wenzel, S.E. Current Concepts of Severe Asthma. J. Clin. Investig. 2016, 126, 2394–2403.
- Fahy, J.V. Eosinophilic and Neutrophilic Inflammation in Asthma: Insights from Clinical Studies. Proc. Am. Thorac. Soc. 2009, 6, 256–259.
- Htet, T.D.; Teede, H.J.; de Courten, B.; Loxton, D.; Real, F.G.; Moran, L.J.; Joham, A.E. Asthma in Reproductive-Aged Women with Polycystic Ovary Syndrome and Association with Obesity. Eur. Respir. J. 2017, 49, 1601334.
- Leon, G.; de Klerk, E.; Ho, J.; Jackman, M.; Reimer, R.A.; Connors, K.E.; Luca, P. Prevalence of Comorbid Conditions Pre-Existing and Diagnosed at a Tertiary Care Pediatric Weight Management Clinic. J. Pediatr. Endocrinol. Metab. 2018, 31, 385–390.
- Maybin, J.A.; Critchley, H.O.D. Progesterone: A Pivotal Hormone at Menstruation: Progesterone and Menstruation. Ann. N. Y. Acad. Sci. 2011, 1221, 88–97.
- Rubio Ravelo, L.; Gago Rodríguez, B.; Almirall Collazo, J.J.; Bell Heredia, L.; Fernández Fernández, L. Comparative Study of Progesterone, Estradiol and Cortisol Concentrations in Asthmatic and Non-Asthmatic Women. Allergol. Immunopathol. 1988, 16, 263–266.
- Davis, C.H. Gynecology and Obstetrics. Vol. II, Chap. 3; W. F. Prior Co. Inc.: Hagerstown, MD, USA; pp. 17–50.
- Nieschlag, E.; Behre, H.M.; Nieschlag, S. Testosterone: Action, Deficiency, Substitution; Cambridge University Press: Cambridge, UK, 2012; ISBN 1-107-01290-2.
- Kouloumenta, V.; Hatziefthimiou, A.; Paraskeva, E.; Gourgoulianis, K.; Molyvdas, P.A. Non-Genomic Effect of Testosterone on Airway Smooth Muscle. Br. J. Pharmacol. 2006, 149, 1083–1091.
- Eliasson, O.; Scherzer, H.H.; DeGraff, A.C. Morbidity in Asthma in Relation to the Menstrual Cycle. J. Allergy Clin. Immunol. 1986, 77, 87–94.
- Shames, R.S.; Heilbron, D.C.; Janson, S.L.; Kishiyama, J.L.; Au, D.S.; Adelman, D.C. Clinical Differences among Women with and without Self-Reported Perimenstrual Asthma. Ann. Allergy Asthma Immunol. 1998, 81, 65–72.
- Leuenberger, P.; Künzli, N.; Ackermann-Liebrich, U.; Schindler, C.; Bolognini, G.; Bongard, J.P.; Brändli, O.; Defila, C.; Domenighetti, G.; Karrer, W.; et al. Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA). Schweiz. Med. Wochenschr. 1998, 128, 150–161.
- Liou, C.-J.; Huang, W.-C. Dehydroepiandrosterone Suppresses Eosinophil Infiltration and Airway Hyperresponsiveness via Modulation of Chemokines and Th2 Cytokines in Ovalbumin-Sensitized Mice. J. Clin. Immunol. 2011, 31, 656–665.
- Espinoza, J.; Montaño, L.M.; Perusquía, M. Nongenomic Bronchodilating Action Elicited by Dehydroepiandrosterone (DHEA) in a Guinea Pig Asthma Model. J. Steroid Biochem. Mol. Biol. 2013, 138, 174–182.
- Wenzel, S.E.; Robinson, C.B.; Leonard, J.M.; Panettieri, R.A. Nebulized Dehydroepiandrosterone-3-Sulfate Improves Asthma Control in the Moderate-to-Severe Asthma Results of a 6-Week, Randomized, Double-Blind, Placebo-Controlled Study. Allergy Asthma Proc. 2010, 31, 461–471.
