- two-dimensional nanomaterials
- cancer theranostics
- tumor
- biomedical imaging
- in vitro and in vivo biological applications
1. Two-Dimensional Theranostic Nanomaterials
- Two-Dimensional Theranostic Nanomaterials
The term "theranostic" refers to a comprehensive effort that integrates diagnostics and therapy in a single nanoplatform [1][2][1,2]. Nanotheranostic harnesses the capabilities of nanotechnology, enhancing therapeutic efficacy and diagnosing ability with a marked difference compared to other currently available diagnoses and therapies, including chemotherapy [3] [3], immunotherapy [4] and radiotherapy [5]. Though numerous classes of theranostic nanomaterials have been developed for cancer treatment, two-dimensional (2D) nanomaterials and their nanocomposites have been reported to exhibit remarkable advantages in cancer diagnosis and therapy owing to their ultrathin planar nanostructure and intriguing physiochemical properties. The ultrahigh specific surface area rendered by the large lateral size and ultrathin thickness, electron confinement without interlayer interactions, and maximum mechanical flexibility are unique properties of 2D nanomaterials, which differentiate them from their bulk counterparts and other types of nanomaterials such as zero-, one-, and three-dimensional networks [6]. The ultrathin planar nanostructure of these 2D bio-nanosystems provides numerous anchoring sites for therapeutic drug molecules.
Among these 2D nanomaterials, MXene [7][8][7,8], transition metal dichalcogenide (TMDC) [9][10][11][12][13][9–13], black phosphorus (BP) [14][15][16][17][14–17], graphene oxide (GO) [18][19][20][21][18–21], manganese dioxide (MnO2) [22][23][24][22–24], and palladium (Pd) [25][26][27][25–27] have attracted tremendous attention in cancer theranostics [28][29][30] [28–30]. Generally, 2D inorganic nanoparticles are synthesized either by bottom-up or top-down approaches. The bottom-up methods include organic ligand-assisted growth, 2D template-confined growth, seeded growth, small molecules and ions mediated synthesis, hydro-/solvothermal methods, crystal phase transformation, biological synthesis, and nanoparticle assembly. Mechanical compression, exfoliation, and nanolithography are the methods of top-down approaches [31].
MXenes, which denote the family of 2D transition metal carbides, carbonitrides, and nitrides, have emerged as potential nanocarriers with the intrinsic property of photothermal-conversion in cancer hyperthermia. The temperature point in the surrounding of MXene is elevated upon external irradiation by a near-infrared (NIR) laser with an excitation wavelength at 808 nm. It has been reported that cancerous cells are more sensitive to heat than normal healthy cells. In this context, MXenes have proved capable candidates in the field of biomedicine [28]. Generally, MXenes are produced by extracting A-element from the layered ternary carbides of MAX phases. M indicates an early transition metal, whereas A denotes an A group element in the periodic table. X can be either C or N. There are reports that MXenes exhibit excellent hydrophilicity, metallic conductivity, and mechanical properties [32][33][32,33]. It was reported that MXenes, including Ti3C2Tx and Nb2CTx produced by a solvothermal treatment, had five times greater surface areas than the MXenes generated by a HF-etching protocol [34]. In 2016, the first nitride MXene was reported, priduced by the reaction of the MAX phase precursor with molten salts at high temperatures [35]. Limbu et al. described a facile and environmentally benign reduced Ti3C2Tx MXene prepared through a simple treatment with L-ascorbic acid at room temperature [36]. TMDCs, including MoS2, MoSe2, WS2, WSe2, and Bi2Se3, consist of hexagonal layers of metal atoms sandwiched between two layers of chalcogen atoms. Several research groups have explored TMDCs as drug delivery platforms and NIR-absorbing agents for cancer combination therapy [37]. Many methods, such as direct solvothermal synthesis, chemical vapor deposition, mechanical and chemical exfoliation, and thermal ablation, have been adopted for the scaled-up production of TMDC nanosheets [38]. TMDCs such as NbS2, MoS2, and WS2 were synthesized by the vapor-phase reaction of their respective metal chloride salts at 800-850 °C [39]. Yu et al. have demonstrated the mechanism of deposition in TMDC systems by studying the removal of MoS2 nanosheets from the precursor MoCl5 in the presence of sulfur gas by the low-pressure chemical vapor deposition reaction [40]. They stated that the rate-limiting step is governed by the relative partial pressure of the MoS2 gas and the vapor pressure of the growing MoS2 NSs. It was proved that mono-, bi- and tri-layer MoS2 nanosheets could be generated if the oxygen plasma treatment is controlled with durations of 90, 120, and 300 s, respectively, prior to its growth [41].
Due to the large surface area by volume and intrinsic high NIR absorbance, nanographene oxide (NGO) nanosheets act as the carrier for photosensitizers and photothermal agents as well [42]. They can load many water-insoluble drugs with π–π hydrophobic interactions. They display remarkable theranostic behavior in combinatorial photodynamic and photothermal (PDT/PTT) treatment under 808 nm laser irradiation and hence have been widely used in cancer treatment [43]. It has also been reported that the mass extinction coefficient of GOs is relatively larger than that of gold nanorods. Owing to the fact they are economically inexpensive, GOs have found many more applications in nanomedicine and electrical devices than carbon nanotubes [44]. Li et al. obtained either a monolayer or a few layers of graphene NSs from worm-like graphite in 1-methyl-2-pyrrolidinone suspension using a facial liquid phase exfoliation procedure [45]. Ramesha et al. produced reduced graphene oxide by reducing the exfoliated graphene oxide, which had a high surface area lacking a high negative surface charge [46]. Graphene oxide produced by the Hummers method from flake graphite was reported to interrupt the conjugation in the graphene plane and produce oxygen-containing functional groups, including epoxide, hydroxyl, and carbonyl on its surface [47][48][47,48]. Exfoliated 2D MnO2 nanosheets with ultrathin thickness were reported to exhibit ultrasensitive responsibility to the tumor microenvironment and release Mn2+ in response to mild acidic conditions. Solid tumors are metabolically different from healthy tissues in many ways. They produce an excessive amount of lactic acid and H2O2 due to the upregulated glycolytic metabolism during tumorigenesis [49][50][49,50]. Hence, the metabolism and excretion of the MnO2 nanosheets could be facilitated during the theranostic tumor treatment [51]. Omomo et al. reported the synthesis of exfoliated layered manganese oxide (H0.13MnO2·H2O) dissolved in tetrabutylammonium hydroxide solution following a top-down approach [52]. Kazuya Kai et al. showed for the first time a single-step bottom-up approach to synthesize MnO2 NSs directly from an aqueous solution of MnCl2 [53].
Being a most stable allotrope of phosphorous, black phosphorous (BP) shows a layer-dependent energy band spanning from a bulk value (0.3 eV) to a monolayer value (2.0 eV). It has been reported that BP nanosheets exhibit a high photothermal conversion efficiency with a significant NIR extinction coefficient. Further, BPNSs are biocompatible as the final degradation products of BP, such as phosphonate and phosphate, are non-toxic [54]. BPNSs possess a large surface area with a folded plane configuration and act as an efficient drug delivery candidate [55]. Owing to their unique optical, electronic, and mechanical properties, BPNSs have found several versatile biomedical applications. As BP NSs generate singlet oxygen species in the entire visible light region, they could be employed for photodynamic therapy [56]. Brent et al. synthesized a few-layered BP NSs for the first time via the liquid exfoliation procedure of BP in N-methyl-2-pyrrolidone [57]. Smith et al. produced BP NSs (>3 μm2) from red phosphorus directly on a silicon substrate using a chemical vapor deposition method [58]. Because of their strong and well-defined near-infrared (NIR) surface plasma resonance properties and high photothermal stability, palladium (Pd) nanosheets and their nanostructures have been widely used as photo-based theragnostic agents [59]. In 2009, Siril et al. synthesized ultrathin Pd NSs with a thickness of about 2 nm purging carbon monoxide for the first time [60]. The literature reports revealed that the thickness of Pd NSs could be limited to less than 10 atomic layers by the influence of CO [61]. The shape of Pd NSs was extremely stable on exposure to NIR radiation compared to silver and gold nanostructures. Pd NSs, exhibiting a thickness of 2.3 nm and an average edge length of 124 nm, were produced by reducing palladium (II) acetylacetonate in the presence of aqueous ascorbic acid solution [62]. Some other 2D nanomaterials based on boron and gold are available with limited biomedical applications [63][64][63,64]. In the present review, we have compiled in vitro and in vivo cancer-treating multifunctional theranostic application of well-explored 2D theranostic nanomaterials. Many reviews have been published, so far, based on 2D theranostic nanomaterials demonstrating various therapies and treatments. They have listed theranostic application of such nanomaterials with respect to biomedical imaging, photothermal conversion efficiency, and dimensional values, including size and thickness of the nanomaterials [65][66][67][65–67]. Some literature showed their biological effects randomly either with in vitro or in vivo treatment [68][65,68]. In the current review, we have presented the theranostic application of 2D nanomaterials collecting only the piece of literature works, which demonstrate both in vitro and in vivo biological effects.
2. Theranostic Properties of 2D Nanomaterials in Cancer Treatment
- Theranostic Properties of 2D Nanomaterials in Cancer Treatment
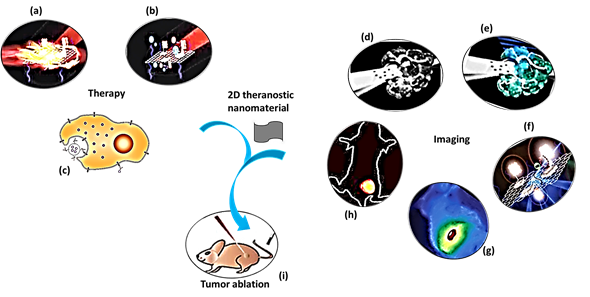
The purpose of theranostic therapy is not only enhancing therapeutic effects but also reducing side effects and improving targeting ability. Due to exceptional theranostic photothermal, photodynamic and chemotherapies, 2D theranostic nanomaterials express magnetic resonance [22], photoacoustic [69] [69], fluorescence [70][71][70,71], and upconversion luminescent imaging ability [72][73][72,73], and sometimes lead to targeted drug delivery [74] (Figure 1). The roles of 2D nanostructured materials in cancer treatment by both in vitro and in vivo studies are listed in Table 1.
Photothermal therapy (PTT) is an emerging treatment method to eradicate cancer tumors, in which light energy is converted into heat energy by using NIR-absorbing agents [75][76][75,76]. The use of NIR light triggers a high temporal and spatial control of local heating, minimizing adverse side effects. Two essential features viz. reduced tissue scattering, and high photothermal-conversion efficiency need to be followed to achieve an effective tumor-tissue ablation during NIR irradiation. NIR light can be further classified into two, i.e. NIR-I and NIR-II bio windows with the wavelength range of 750–1000 nm and 1000–1350 nm, respectively [77][32,77]. During NIR-triggered photothermal treatment, local hyperthermia is produced elevating temperature of the tumor microenvironment more than 42 °C, which is enough to ablate the tumor. Also, PTT bears more significant advantages with minimal invasiveness and a high selectivity than conventional cancer treatment strategies, including simple operation [78].
Figure 1. A schematic diagram showing theranostic treatments of 2D theranostic nanomaterials such as photothermal therapy (a), photodynamic therapy (b) and chemotherapy (c) with their astonishing imaging properties, including magnetic resonance (d), photoacoustic (e), fluorescence (f), infra-red thermal (g), and upconversion luminescence imaging (h) to eradicate cancer tumor (i) effectively. The images (a), (b), (f), and (i) were adapted with permission from [79]. The image (c) was adapted with permission from [80]. The images (d) and (e) were adapted with permission from [81]. The image (g) was adapted with permission from [37]. The image (h) was adapted with permission from [82] [82].
Photodynamic therapy (PDT) is an alternative high-efficient cancer treatment approach, which generates a large amount of singlet oxygen (1O2) from activatable photosensitizers (PS) and subsequently causes apoptosis or necrosis of cells [83][84][85][83–85]. Though PTT and PDT therapies are efficient in cancer treatment, some drawbacks are encountered. PTT, sometimes, causes non-specific cellular damage to healthy tissues because a few portions of NIR laser light travel into some healthy tissues while treating tumor tissues. Likewise, the therapeutic effects of PDT are also limited due to the insufficient oxygen supply and limited penetration depth of visible or ultraviolet light sources. In such circumstances, the impact on tumor growth of a single treatment by either PTT or PDT becomes unsatisfactory. Hence, the researchers introduced the integration of PTT and PDT into one system to improve the therapeutic efficacy of studied nanomaterials [38]. Whenever PSs are administered for the PDT treatment, PSs are retained in tumor tissues at a large quantity because the tumor cells possess an inadequate lymphatic system. In contrast, healthy tissues eliminate them over time. Hence, localized activation by NIR irradiation makes PDT a selective treatment for killing cancer cells. The localized oxidative photodamage triggered by the therapy induces three main mechanisms of cell death at the tumor site. They are apoptosis, necrosis, and autophagy, which are accompanied by the induction of an acute local inflammatory reaction to remove dead cells and restore healthy tissue. PDT is a highly controllable therapy owing to a short-range action of singlet oxygen (1O2) with a lifetime of ∼40 ns [86]. Apart from NIR-mediated PDT, alternative strategies such as X-ray PDT and sonodynamic PDT were also developed by the researchers to treat cancer. Sonodynamic therapy (SDT) could activate a sonosensitizer through the sonoluminescence process, pyrolytic reaction, or acoustic cavitation effects [87]. The advantageous property of SDT over PDT is the higher tissue penetration depth [88]. In contrast to conventional PDT, X-ray PDT is essentially a combination of RT and PDT, the key factors of which include X-ray dose, the concentration of O2, and the efficiency of the intersystem crossing. X-ray PDT is effective in causing oxidative degradation of unsaturated lipids and surface proteins, short-term cell necrosis, and DNA damage [89][90][91][92][93][94] [89].
Tumor imaging technologies, including magnetic resonance (MR) imaging and photoacoustic (PA) imaging, are related to theranostic therapies, which are helpful for the accurate diagnosis of cancer. MR images are clear with subtle changes due to their excellent resolving power in soft tissues. In contrast, PA imaging produces sharp contrast tissue images as a non-invasive and non-ionizing biomedical imaging technique. Some researchers combine different imaging methods to achieve accurate and early diagnosis of cancer [78]. As photosensitizers are susceptible to photo-bleaching and self-destruction upon prolonged light exposure, the development of novel PS nanocomplexes without fluorescence quenching is necessary for theranostic treatment approaches [79]. Upconversion luminescence (UCL) imaging for tumor cells has attracted substantial attention in recent years owing to the unique properties of upconversion nanomaterials, which minimize the background interference from autofluorescence of biosamples and improve tissue penetration. The upconversion method is an anti-Stokes process whereby two or more low-energy photons from NIR light are absorbed to emit higher energy in the visible region [42]. Sophisticated imaging techniques, including computed tomography, positron emission tomography (PET), and X-rays, are inevitable in cancer diagnosis. When the treatment target is not identified, radiotherapy provides required inputs from imaging for planning the treatment [90].
2D theranostic nanomaterials undergo easy surface modification and hence possess a high drug loading capacity for numerous small-molecule anticancer drugs, enzymes, and therapeutic genes. For instance, PEGylated graphene oxide reported by Liu et al. delivered hydrophobic anticancer drug SN38 with therapeutic efficacy than that of FDA-approved SN38 prodrug [91]. The reasons are the specific interactions such as π–π stacking and hydrophobic forces of aromatic ring-containing anticancer drug molecules with the graphene nanosheets. In the acidic environment of cancer tumors, the nanomaterials release the drug molecules due to the protonation effect [92]. Tao et al. conjugated black phosphorus nanosheets with amine-terminated PEG, whereby the loading capacity of DOX was significantly high by 108% w/w. Further, they demonstrated that the interaction between DOX and the black phosphorus could be disrupted by protonation or hyperthermia [15]. The specificity of drug action gains paramount importance in cancer treatment to ensure the minimization of any toxic effects on healthy cells. It was discovered that cancers could be hematologic or solid tumors, and hence different strategies need to be developed for each type of cancer. The distinctive pathophysiological features of tumor tissue are helpful for targeted drug delivery. Tumor-associated antigens (TAAs) such as folate, low-density lipoprotein, and gonadotropin/luteinizing hormone-releasing hormone receptors are the specific proteins, which are highly expressed over the cancer cells [93][94][93,94]. When TAAs are conjugated with theranostic 2D nanomaterials, they would apparently enhance tumor therapy, imaging, and drug delivery along with tumor specificity.