Cadmium (Cd) is an extremely toxic heavy metal released in the environment due to a plethora of anthropic processes. . Here focused on the effects of two sublethal cadmium concentrations on cells of the gastrointestinal surface epithelium with particular attention to changes in the distribution of glycan residues and the morphological features of the intestine. To evaluate the defense response of the mucosa, the expression of metallothionein was investigated. Data demonstrate that cadmium modifies the presence and/or distribution of glycans in the brush border and cytoplasm of enterocytes and in the goblet cells cytoplasm. Results suggest a significant interference of cadmium, in dose and site-dependent manner, with mucosal efficiency. This effect could be a direct health risk for the organism exposed to the contamination and indirectly a risk for the trophic chain.
1. Introduction
Cadmium (Cd) is an extremely toxic heavy metal released in the environment due to a plethora of anthropic processes. By inducing extensive cellular damage, it causes deleterious effects on animal behavior and on adult and embryonic tissues
[1][2], as well as altering gene expression
[3][4][5]. The liver and kidney, the two main detoxifying organs, are particularly affected, but harmful effects have been reported on many targets, including the retina, brain, and muscles
[6][7][8][9][10]. Cadmium is also an endocrine disruptor interfering with fecundity, oogenesis, and spermatogenesis
[11][12]. Cadmium toxicity is higher in the aquatic organisms compared with land animals. In fact, in the former case exposure occurs from ovo to death and the route of absorption is particularly large, being represented by the skin and the gills. Both organs are markedly affected by the metal that causes severe morphological and molecular alterations. A further route of absorption is the gastrointestinal tract through which contaminated water and/or contaminated preys are ingested, allowing metal distribution to the entire body via the bloodstream. In the gastrointestinal tract, cadmium compromises the integrity of the microbiota, thus reducing the efficiency of this intestinal barrier and increasing the risk of inflammation, allergies, and metabolic disorder. Cadmium interferes with the gut mucosa: degenerated nuclei, apoptosis,
hydropic degeneration, swelling, necrosis and altered lamina propria infiltrated with leukocytes. Cadmium also affects another important component of the gut: the goblet cells. Goblet cells are cells of the gastrointestinal surface epithelium specialized in the synthesis of mucus, a gel consisting of water, ions, and specific glycoproteins, termed mucins. The mucus coating the gastrointestinal tract is considered the front line of the innate host defense, being a pivotal player of the immune system. In the small intestine, mucus limits the number of bacteria that can reach the epithelium; in the large intestine, the inner mucus layer separates the commensal bacteria from the host epithelium. The goblet cells also have a role in the presentation of oral antigens to the immune system. The intestinal goblet cells secrete not only mucins but also several typical bioactive molecules; the observation that mucus defects lead to diseases such as inflammatory bowel disease and or hyperglycemia underlines the importance of this barrier. Changes in the expression of mucus genes and in mucin glycan structures occur in cancers of the intestine, contributing to diverse biologic properties involved in the development and progression of cancer. Cadmium has been shown to damage the intestinal mucosal barrier making the body more susceptible to infection and inflammation. This aspect is of particular concern in fish aquaculture, where the animals are fed with flour often derived from fish of little commercial value from particularly polluted areas. This can greatly increase the occurrence of infections in farmed fish, resulting in increased use of drugs and antibiotics which, in turn, further reduce the quality of farmed fish.
2. Effects of Waterborne Cadmium Exposure on Zebrafish intestine
2.1. Effects of Cadmium on Gut Morphology
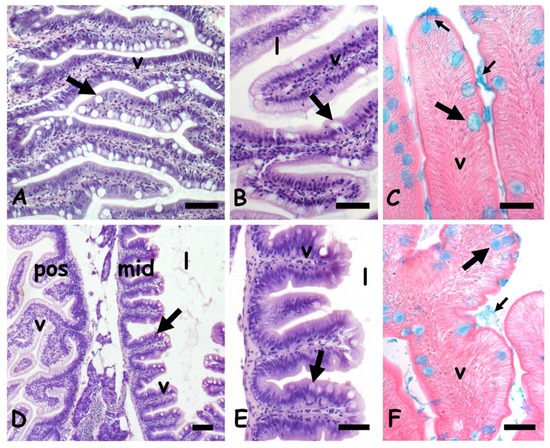
In control zebrafish, the anterior intestine shows elongated villi covered by a columnar mucosa in which cylindrical enterocytes are interspersed with large and round goblets cells (
Figure 1A,B). Their mucous content is clearly stained by Alcian blue (
Figure 1C). In the mid intestine, the villi are broad and short and the lumen relatively large (
Figure 1D,E). Mucosa goblet cells are numerous and clearly stained by Alcian blue (
Figure 1F).
Figure 1. Danio rerio anterior (A–C) and mid (D–F) intestine. (A) Long villi (v) lined by a mucosal epithelium rich in goblet cells (arrow). (B) Detail of villi (v); lumen (l), goblet cell (arrow). (C) Goblet cells (large arrow) and luminal mucus (small arrow) positive to the Alcian blue staining. (D) Villi (v), goblet cells (arrow), and lumen (l) in posterior (pos) and mid (mid) gut. (E) Detail of midgut villi. (F) Goblet cells (large arrow) and luminal mucus (small arrow) positive to the Alcian blue staining. Hemalum-eosin staining (A,B,D,E); Alcian blue-eosin counterstaining (C,F). Bars: 100 µm (A,B,D), 50 µm (C,E,F).
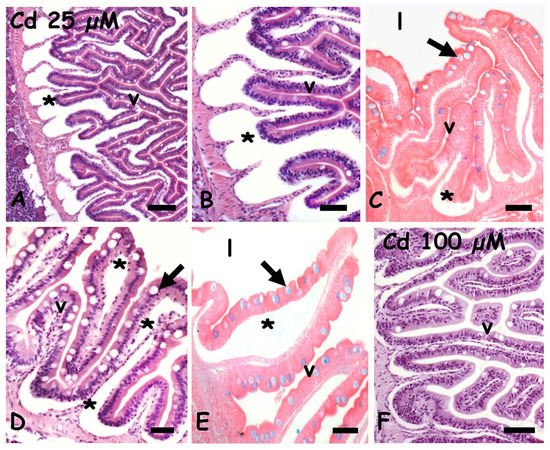
After exposure to 25 µM of cadmium, in both anterior (
Figure 2A,B) and mid (
Figure 2D) intestine, the mucosa is detached from the basal connectives by evident seric infiltrations extending in the lamina propria. The mucosal epithelium is apparently intact but goblets cells positivity to the Alcian blue is significantly reduced (
Figure 2C,E). Exposure to 100 µM cadmium apparently has a far less toxic effect: the seric infiltrates are less relevant (
Figure 2F) and the goblet cells are clearly stained by Alcian blue (data not shown).
Figure 2. Danio rerio anterior (A,C,F) and mid (D,E) intestine exposed to waterborne cadmium. (A) Seric infiltrates (*) between connectives and mucosal epithelium. Villi (v). (B) Detail. (C) Poorly stained goblet cells (arrow) in villi (v) with seric infiltrates (*); gut lumen (l). (D) Seric infiltrates in lamina propria (*) and goblet cells (arrow). (E) Poorly stained goblet cells (arrow) in villi (v) showing extensive seric infiltration (*); gut lumen (l). (F) Intact villi (v). Hemalum-eosin staining (A,B,D,F); Alcian-blue-eosin counterstaining (C,E). Bars: 150 µm (A), 100 µm (B,C,F), 50 µm (D,E).
2.2. Effects of Cadmium on Metallothionein (MT) Expression
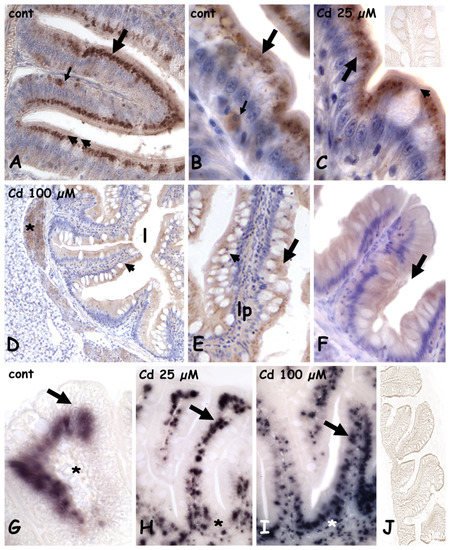
In controls (
Figure 3A) and in animals exposed to 25 µM of cadmium, in both anterior (
Figure 3B,C) and mid (
Figure 3A) intestine, MT is abundant in the apical cytoplasm of the cylindrical enterocytes and in occasional sparse cells located close to the connectival lamina propria. Goblet cells and their content are always unstained (
Figure 3A,C). After exposure to 100 µM of cadmium, in both anterior (
Figure 3D) and mid (
Figure 3E,F) gut, labeling is much less intense, and MT is uniformly distributed in the cytoplasm of enterocytes. No intensely labeled basal cells are observed, and goblet cells are always unstained (
Figure 3E,F), similar to negative controls (
Figure 3C inset).
Figure 3. Metallothionein (A–F) and metallothionein mRNA (G–J) localization in the anterior and mid intestine in Danio rerio exposed to cadmium. (A–C) anterior gut; MT is in the apical cytoplasm of enterocytes (large arrow) and in occasional cells located close to the lamina propria (small arrow). Goblet cells are unstained (arrowheads). Anterior gut (D,E) and mid (F) gut; MT is uniformly dispersed in the cytoplasm of enterocytes (arrow). Goblet cells (arrowheads) are unstained. Lamina propria (lp), positive pancreatic cells (*). Inset in (C): negative control of immunocytochemistry. (G) anterior gut; MT mRNA is in enterocytes nuclei (arrow). Lamina propria is unstained (*). (H–I) anterior gut; dose-dependent increase in labeled submucosa and lamina propria nuclei (arrows). (J) Negative control of in situ hybridization. Hemalum counterstaining (A–F).
MT messengers in the control gut are in the nuclei of enterocytes, while the lamina propria is negative (
Figure 3G). After Cd exposure (
Figure 3H,I), many small, labeled nuclei appear in the submucosa and in the lamina propria and their number is clearly dependent on metal dose. Negative controls are always unstained (
Figure 3J).
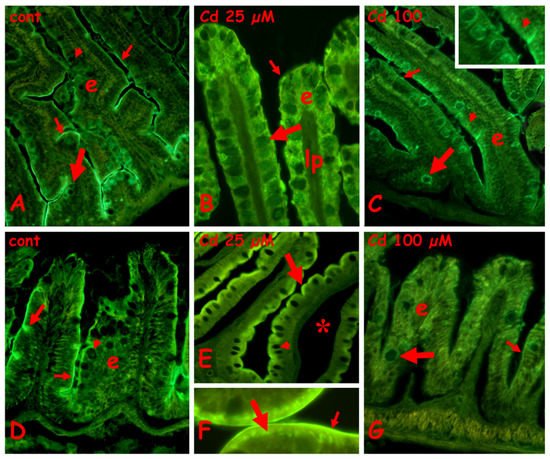
2.3. Effects of Cadmium on the Carbohydrate Composition of Gut Cells
2.3.1. N-Acetyl-Glucosamine Staining with Fluorescent WGA Lectin
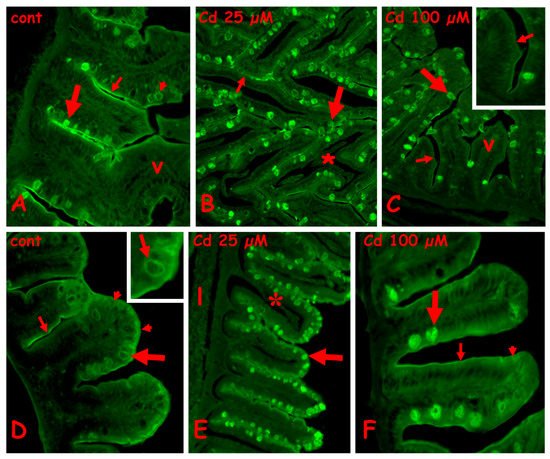
In the control anterior gut, labeling is present on the brush border, the mucus of the goblet cells is located basally in the villi, and the cytoplasm of the goblet cells is located apically (
Figure 4A). After exposure to 25 µM (
Figure 4B) or 100 µM of cadmium (
Figure 4C), in the anterior gut, all the goblet cells are labeled, on both cytoplasm and mucus vesicles. The brush border remains labeled after exposure to 25 µM of cadmium (
Figure 4B), but after 100 µM of cadmium (
Figure 4C), it shows long tracts completely negative to the lectin. The enterocytes are always unlabeled (
Figure 5A–C).
Figure 4. Effects of cadmium exposure on gut cells carbohydrate composition: staining for N-acetyl-glucosamine with fluorescent lectin WGA. Anterior (A–C) and mid (D–F) gut. (A) Villi (v) with labeled brush border (small arrow) and mucus vesicles in basolateral goblet cells (large arrow). In apical goblet cells labeling is cytoplasmic (arrowhead). (B) Intensely labeled apical goblet cells (large arrow) and brush border (small arrow). Seric infiltrate (*). (C) Labeled goblet cells in intact villi (v). Unstained brush border (small arrows). (D) Labeled brush border (small arrow), apical cytoplasm of enterocytes (arrowheads) and cytoplasm of goblet cells (large arrow and inset). (E) Labeled goblet cells (arrow) in villi with seric infiltrates (*). Unstained liver (l). (F) Labeled mucus vesicles in basal goblet cells (large arrow) and brush border (small arrow). Diffuse staining in the apical cytoplasm of enterocytes (arrowhead).
Figure 5. Effects of cadmium exposure on gut cells carbohydrate composition: staining for galactose with fluorescent lectin PNA. Anterior (A–C) and mid (D–F) gut. (A) Unlabeled enterocytes (e), goblet cells (large arrow), and brush border (small arrow). (B) Labeled brush border (small arrow) and scattered epithelial cells located at the base of the epithelium (large arrow). Note the seric infiltrate (*) and the unlabeled goblet cells (arrowheads). (C) Scattered labeled epithelial cells (large arrow) among unlabeled enterocytes (e), goblet cells (arrowhead) and brush border (small arrow). (D–F) Labeled cells (large arrows) in basal epithelium. Unlabeled enterocytes €, brush border (small arrow), and goblet cells (arrowhead). In (F), note the abundance of labeled cells.
In control midgut (
Figure 4D), a diffuse staining is observed on the apical cytoplasm of enterocytes, on the brush border, and on the cytoplasm of the goblet cells (
Figure 4D inset). After exposure to 25 µM of cadmium, all the goblet cells have labeled mucus granules (
Figure 4E), while after exposure to 100 µM of cadmium, only the basal goblet cells are intensely labeled (
Figure 4F). The enterocytes’ cytoplasm appears diffusely labeled and the brush border appears discontinuous (
Figure 4E,F).
2.3.2. Galactose Staining with Fluorescent PNA Lectin
In the control anterior gut, the villi are completely unlabeled (
Figure 5A). After exposure to 25 µM of cadmium (
Figure 5B), an intense labeling is present on the brush border and on scattered epithelial cells located close to the connectival lamina propria. The enterocytes show a moderate and diffuse apical positivity, while the goblet cells are completely unlabeled. A similar result is obtained in villi exposed to 100 µM of cadmium, in which, however, labeled cells are more numerous (
Figure 5C).
In control midgut (
Figure 5D), labeling is present on scattered epithelial cells located close to the lamina propria; enterocytes and goblet cells are unstained. After exposure to 25 µM (
Figure 5E) or 100 µM of cadmium (
Figure 5F), the labeled cells become more numerous, but no labeling appears on enterocytes, brush border, or goblet cells.
2.3.3. Staining for N-Acetyl-Galactosamine with Fluorescent RCA Lectin
In control anterior gut, labeling is present on the brush border and on the cytoplasm of basal goblet cells. All the enterocytes and the goblet cells located along the villi are completely unlabeled (
Figure 6A). After exposure to 25 µM of cadmium (
Figure 6B), a diffuse staining is present on the enterocytes and, to a lesser extent, on mucus vesicles of goblet cells. In villi exposed to 100 µM cadmium, staining is on the cytoplasm of the enterocytes and goblet cells (
Figure 6C and inset) and on the brush border.
Figure 6. Effects of cadmium exposure on gut cells carbohydrate composition: staining for N-acetyl-galactosamine with fluorescent lectin RCA. Anterior (A–C) and mid (D–F) gut. (A) Villi with labeled brush border (small arrows) and cytoplasm of basal goblet cells (large arrow). The enterocytes (e) and the goblets cells located in the apical portion of villi (arrowhead) are unlabeled. (B) Diffuse labeling on enterocytes (e), brush border (small arrow), and goblet cells (large arrow). Unlabeled lamina propria (lp). (C) Labeled goblet cells cytoplasm (large arrow) and brush border (small arrow); enterocytes € show a moderate apical positivity (arrowhead and inset). (D) Labeled brush border (small arrow) and mucus vesicles in occasional goblet cells (large arrow); unlabeled enterocytes (e) and goblet cells (arrowhead). (E,F) Labeled apical cytoplasm of enterocytes (large arrow) and brush border (small arrow). Unlabeled goblet cells (arrowhead), seric infiltrate (*). (F) Detail of (E). (G) Unstained enterocytes (e), goblet cells (large arrow), and brush border (small arrow).
In control midgut, labeling is present on the brush border and on the mucus vesicles of occasional goblet cells (
Figure 6D). After exposure to 25 µM of cadmium, the apical cytoplasm of enterocytes appears intensely labeled (
Figure 6E) due to the formation of a series of very fluorescent and regularly disposed dense bodies (
Figure 6F). The brush border is also intensely labeled (
Figure 6F). After exposure to 100 µM of cadmium, the villi are completely unlabeled (
Figure 5G).
3. Conclusion
In summary, here demonstrated that cadmium affects the gut at different level. First, histological analysis shows cadmium-induced villar degeneration and seric infiltration. Exposure to cadmium, regardless of the dose, significantly interferes with the presence and/or distribution of glucids in the different cell types at different levels of the gut. Conversely, changes in MT expression and localization are dose-dependent, being detectable only after the exposure to the higher dose. Although the relationship between changes in mucus lectins and the well-known changes in the intestinal microbiota induced by cadmium remains speculative, it is conceivable that the glucidic components of mucins play a key role in such toxic mechanism, hence further studies on the chemical properties of mucins will help us to understand it. Taken together, these findings highlight the health risk of environmental and dietary exposure to cadmium for animals and people living in highly polluted habitats.