Salvia miltiorrhiza Bunge, also known as red sage, is a valued herbal plant in the traditional medicine in Korea, China and Japan. It is called as Dansam in Korea, Danshen in China. It is well known for its highly medicinal properties in treating of heart and vascular diseases, chronic renal failure, Alzheimer’s disease, hepatitis and so forth.
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body.
- Salvia miltiorrhiza Bunge
- dansam
- cancer
- cardiovascular diseases
- liver diseases
- nervous system diseases
- anti-inflammation
- antioxidant
1. Introduction
Salvia miltiorrhiza Bunge (S. miltiorrhiza), also known as dansam in Korean and danshen in Chinese, has been used for the treatment of cardiovascular and cerebrovascular diseases, especially in Asia [1]. It is a deciduous perennial plant which belongs to genus Salvia of Lamiaceae family [2]. The active components of S. miltiorrhiza are divided into two groups, one of which is water-soluble phenolics including salvianolic acid A (Sal A), salvianolic acid B (Sal B), lithospermic acid, rosmarinic acid, and R-(+)-β-(3,4-dihydroxyphenyl)lactic acid, named danshensu [3] and the other is lipophilic tanshinones including tanshinone I, tanshinone IIA, tanshinone IIB, cryptotanshinone, and dihydrotanshinone I [4]. Salvianolic acids (including Sal A and Sal B), the most abundant compounds from S. miltiorrhiza, are known to exhibit diverse biological activities such as antioxidant [5], anti-inflammatory [6], antithrombotic [7], and cardioprotective activities [8,9], while tanshinones show antitumor [10], cardioprotective [11], neuroprotective and analgesic activities [12], and so forth.
Dried roots of S. miltiorrhiza, extracted with various solvents, have been reported to have therapeutic effects on a variety of diseases including cancers [13], cardiovascular diseases [14], liver diseases [15], and nervous system diseases [16]. So far, there have been a small number of review articles on S. miltiorrhiza extract, most of which focused on its therapeutic use on a certain disease, mainly cardiovascular diseases [17,18] or Alzheimer’s disease [19]. Given its wide usage on many different diseases, there is a need to review studies addressing therapeutic effects and mechanisms of action of S. miltiorrhiza in different groups of diseases.
2. Cancer and S. miltiorrhiza
Several studies about the anti-cancer effect of S. miltiorrhiza have been reported (Table 1). According to Kim et al., treatment with 70% ethanol extract of S. miltiorrhiza inhibited matrix metalloproteinase (MMP)-9 expression and cell invasion in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced MCF-7 breast cancer cells, possibly through down-regulating the mitogen-activated protein kinase (MAPK)/activator protein-1 (AP-1) signaling pathway [30]. Treatment with S. miltiorrhiza extract at a dose of 50 µg/mL for 24 h decreased the phosphorylation of MAPKs, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. In addition, it down-regulated phospho (p)-c-Jun expression, implying that S. miltiorrhiza extract had an inhibitory effect on TPA-induced MMP-9 expression through blocking the activation of the transcription factor AP-1, a dimer consisting of either Jun/Jun homodimers or Fos/Jun heterodimeric complexes. Wu et al. found that an herbal mixture extract named CASE (Astragalus and Salvia miltiorrhiza water/ethanol extract [71:1.85]) suppressed hepatocellular carcinoma (HCC) progression in vivo (Diethylnitrosamine [DEN]-induced HCC in rats) and in vitro (TGF-β1-stimulated HepG2 cells) [31]. At first, 60, 120, or 240 mg/kg of CASE was orally administered to Sprague Dawley (SD) rat with DEN-induced HCC for 28 days, which increased relative microRNA (miR)-145 expression but decreased relative miR-21 expression. In vivo, BALB/c mice xenografted with HepG2 cells were intragastrically injected with 310 mg/kg of CASE once every four days for 28 days, which reversely regulated miR-145 antagomir/miR-21 agomir-mediated Smad3 phosphorylation. CASE up-regulated miR-145 and p-Smad3C expression and down-regulated miR-21, p-Smad3L, p-ERK1/2, p-JNK1/2, and p-p38 expression. According to Boye et al., the same herbal mixture extract (CASE) also affected MAPK-regulated TGF-β/Smad signaling in HCC [32]. In an in vivo mouse model of DEN-induced HCC, administration of CASE at doses of 60, 120, or 240 mg/kg for 12 or 16 weeks suppressed p-ERK, p-JNK, and p-p38 expression. On the other hand, in vivo pretreatment of hepatic stellate cells (HSCs) and/or HepG2 cells with CASE at doses of 20, 40, or 80 µg/mL for 24 h before their stimulation with TGF-β1 decreased p-ERK and p-JNK expression, while it increased p-p38 expression. Pretreatment with CASE also concentration-dependently decreased TGF-β1-induced phosphorylation of oncogenic pSmad3L in HSC and HepG2 cells; reduced nuclear import of Smad4; enhanced the phosphorylation of tumor suppressor pSmad3C especially in HepG2 cells; reduced the expression and nuclear relocation of importins (Imp) 7/8; and suppressed plasminogen activator inhibitor (PAI)-1 gene expression. In Kim et al.’s study, after treatment of U266 (human multiple myeloma) and U937 (human myeloid leukemia) cells with 99.9% ethyl alcohol extract of S. miltiorrhiza at doses of 25, 50, 100, or 200 µg/mL for 24 h, cell viability was inhibited in a dose-dependent manner [33]. Further, treatment with S. miltiorrhiza extract at doses of 25 or 50 µg/mL for 24 h increased reactive oxygen species (ROS) generation; enhanced phosphorylation of activating transcription factor 4 (ATF4), eukaryotic initiation factor 2 (eIf2), and protein kinase RNA-like endoplasmic reticulum kinase (PERK); and increased CCAAT-enhancer-binding protein homologous protein (CHOP) activation and cleavage of poly ADP-ribose polymerase (PARP) and caspase-3. In addition, 24 h treatment with S. miltiorrhiza extract significantly elevated the expression of tumor suppressor miR-216b at a dose of 50 µg/mL, whereas it down-regulated its target protein, c-Jun, at doses of 25 or 50 µg/mL. According to Ye et al., methanol extract of S. miltiorrhiza could inhibit the growth of non-small cell lung cancer (NSCLC) via induction of apoptosis through mitochondrial apoptotic pathway and phosphatase and tensin homolog (PTEN)-mediated inhibition of the phosphoinositide 3 kinase (PI3K)/Akt pathway [34]. In vitro treatment with S. miltiorrhiza extract at doses of 20 or 40 µg/mL for 24 h induced apoptosis in Glc-82 cells as observed with Annexin V-FITC/PI staining and up-regulated the expression levels of cleaved caspase-3, -9, and PARP1, suggesting the involvement of the mitochondrial apoptotic pathway. In addition, it increased the expression of Bcl-2-associated X protein (Bax) and the tumor-suppressor proteins p53 and p21, while it decreased the expression of B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xl), both of which are anti-apoptotic components of Bcl-2 family. Further, treatment with S. miltiorrhiza extract also inhibited the phosphorylation of Akt and increased the activity of its upstream inhibitor, PTEN. In vivo, administration of S. miltiorrhiza extract at a dose of 40 mg for 22 days suppressed the growth of lung cancer Glc-82 xenografts in Balb/c mice. Lee et al. demonstrated that the arsenic herbal mixture PROS (tetraarsenic hexoxide [PR] + Olendlandia diffusa and Salvia miltiorrhiza extract (5:2) [OS]) showed apoptotic and anti-angiogenic effects in non-small-cell lung cancer cells (NSCLCs) via inhibition of signal transducer and activator of transcription 3 (STAT3)/vascular endothelial growth factor (VEGF)/cyclin-dependent kinase 2 axis signaling [35]. Treatment with PROS (2.5 µg/mL PR + 180 µg/mL OS) for 24 h exerted significant cytotoxic effects on A549 or H460 better than PR or OS alone and induced apoptosis and S phase arrest, as assessed by DAPI staining and cell cycle analysis. Further, PROS treatment decreased the phosphorylation of STAT3, ERK, proto-oncogene Src, Akt, cyclooxygenase 2 (COX-2), and suppressor of cytokine signaling 1 (SOCS-1) and subsequently inhibited the binding of STAT3 with VEGF or CDK2. PROS also inhibited VEGF-induced proliferation, migration, and tube formation in human umbilical vein endothelial cells (HUVECs) and ex vivo angiogenesis in chick chorioallantoic membranes (CAMs). PROS treatment down-regulated the phosphorylation of VEGFR2, Src, and STAT3 in HUVECs. In the H460 xenograft model, subcutaneous injection of PROS down-regulated STAT3 and VEGF expression and caspase-3 activation. In Wang et al.’s study, either double-distilled water (ddH2O), 95% ethanol, or 1:1 water/ethanol extract of S. miltiorrhiza was added to two human oral squamous cell carcinoma (OSCC) cell lines, HSC-3 and OC-2, and it turned out that 95% ethanol extract of S. miltiorrhiza showed the greatest antioxidant and radical scavenging capabilities [36]. After treatment of HSC-3 cells with S. miltiorrhiza alcohol extract at doses of 10, 25, or 50 µg/mL for 48 or 72 h, significant decreases in the expression of X-linked inhibitor of apoptosis protein (XIAP) and survivin were observed but there were no changes in the levels of mitochondrial membrane potential (ΔΨm), antiapoptotic proteins (Bcl-2 and Bcl-xL), and proapoptotic proteins (Bax and Bcl-2 associated agonist of cell death [Bad]). In vivo, BALB/c NU mice xenografted with HSC-3 tumor were intraperitoneally injected with 50 or 100 mg/kg of S. miltiorrhiza extract for 34 days, which resulted in the suppression of tumor growth without any significant impacts on mouse body weights; as for biological markers, treatment with S. miltiorrhiza extract led to decreased expression of XIAP and survivin but not Bcl-2 family members. Yang et al. examined the antiproliferative effect of S. miltiorrhiza extract on oral cancer cells [37]. In vitro, 95% ethanol extract of S. miltiorrhiza was added to three OSCC cell lines SAS, SCC25, and Oec-ml (at doses between 0–30 µg/mL for 24 h) and six KB drug-resistant OSCC cell lines (at doses between 0–80 µg/mL for 24 h). In vivo, oral cancer SAS xenograft mice were administered with S. miltiorrhiza extract at a dose of 10 mg/kg for 32 days. Treatment with S. miltiorrhiza extract, both in vitro and in vivo, induced apoptosis, as evidenced by the increased active caspase-3 expression and decreased XIAP expression. According to Lee et al., acetonitrile extract of S. miltiorrhiza prevented the progression of prostate cancer cells through the generation of intracellular ROS generation [38]. Treatment with S. miltiorrhiza extract at doses of 5, 20, or 100 µg/mL for 24 or 48 h dose-dependently inhibited the growth of three prostate cancer cell lines (PC-3, LNCap, and DU-145) as measured by trypan blue assay. In addition, treatment with S. miltiorrhiza extract at a dose of 20 µg/mL for 24, 48, or 72 h induced cell cycle arrest at G1/S phase in PC-3 cells; it increased the protein expression of the cyclin-dependent kinase inhibitor p21 and decreased the protein expression of cyclin-dependent kinase 2 (CDK2), CDK4, and cyclin D1 protein. It also induced apoptosis in PC-3 cells as determined by TUNEL assay; it decreased the expression of anti-apoptotic Bcl-2 protein and increased the protein expression of apoptotic inducers such as caspase-9, caspase-3, and PARP. In vitro, PC-3 xenograft mouse model was injected with S. miltiorrhiza extract at doses of 100 mg/kg for 6 weeks. Both in vitro and in vivo, intracellular ROS generation was increased, which was considered to mediate the cytotoxic effect on prostate cancer cells. Sung et al. reported that 100% ethanol or 100% acetone extract of S. miltiorrhiza showed cytotoxicity against human cancer cell lines [39]. After treatment of AGS, A549, HCT116, LNCaP, and MCF-7 cells with S. miltiorrhiza extract (at doses of 5, 10, 20, 40 µg/mL for 24 h), p-JNK, p-ERK1/2, p-p38, cleaved-caspase-3, -7, -9, and cleaved poly ADP-ribose polymerase (c-PARP) expression were elevated. In contrast, it decreased the expression of nuclear p65, thereby inhibiting the progression of cancer cells. Wu et al. demonstrated that dichloromethane-methanol (1:1) extract of S. miltiorrhiza induced intrinsic apoptosis in various drug-sensitive and multidrug-resistant cancer cells [40]. Treatment with S. miltiorrhiza extract at doses of 3, 10, or 30 µg/mL for 1 h induced ROS production in CCRF-CEM cells. They also reported that S. miltiorrhiza extract induced apoptosis through caspases and a PARP-dependent pathway, as evidenced by the increased levels of cleaved caspase-3, -7, -9 and PARP upon treatment with S. miltiorrhiza extract at doses between 5–40 µg/mL. After treatment of CCRF-CEM cells with S. miltiorrhiza extract at a dose of 20 µg/mL for 24 h, TNF-α-induced translocation of p65 from cytoplasm to the nucleus was inhibited; when treated for 2 h, p-JNK, p-ERK1/2, and p-p38 expression were up-regulated. Therapeutic targets of S. miltiorrhiza in cancers are elucidated in Figure 1.
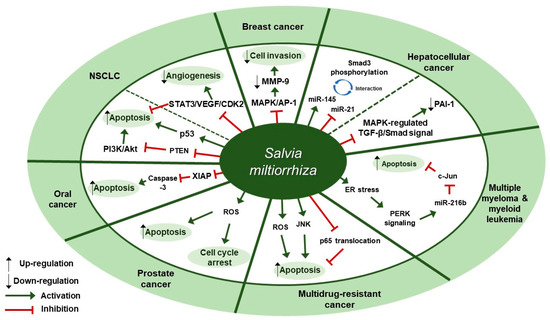
Figure 1. Therapeutic targets of S. miltiorrhiza in cancers. NSCLC, non-small cell lung cancer; MAPK, mitogen-activated protein kinase; AP-1, activator protein-1; MMP-9, matrix metalloproteinase-9; miR-145, microRNA-145; miR-21, microRNA-21; PAI-1, plasminogen activator inhibitor 1; ER, endoplasmic reticulum; PERK, protein kinase RNA-like endoplasmic reticulum kinase; miR-216b, microRNA-216b; JNK, c-Jun N-terminal kinase; ROS, reactive oxygen species; XIAP, X-linked inhibitor of apoptosis protein; PTEN, phosphatase and tensin homolog deleted on chromosome ten; PI3K, phosphoinositide 3-kinase; NSCLC, non-small cell lung cancer; STAT3, signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor; CDK2, cyclin-dependent kinase 2.
Table 1. Cancer and S. miltiorrhiza.
| Disease | Extract | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | 70% ethanol | MCF-7 | 50 µg/mL; 24 h | Inhibition of breast cancer cell invasiveness | ↓ MMP-9, p-ERK, p-JNK, p-p38, p-c-Jun | [ | 30 | ] | ||
| Hepatocellular carcinoma | Astragalus and Salvia miltiorrhiza water/ethanol extract (71:1.85) | (1) SD rat (2) TGF-β1-stimulated HepG2 (3) BALB/c xenograft mouse model |
(1) 60, 120, 240 mg/kg; 28 days (2) 20, 40, 80 µg/mL; 12, 24 h (3) 310 mg/kg; 28 days |
Inhibition of hepatocellular carcinoma progression | ↑ Smad3C, miR-145 ↓ Smad3L, miR-21, p-ERK, p-JNK, p-p38 |
[ | 31 | ] | ||
| Hepatocellular carcinoma | Astragalus and Salvia miltiorrhiza water/ethanol extract (71:1.85) | (1) SD rat (2) HSCs, HepG2 |
(1) 60, 120, 240 mg/kg; 12, 16 weeks (2) 20, 40, 80 µg/mL; 24 h |
Inhibition of hepatocellular carcinoma | (1) ↑ pSmad3C ↓ p-ERK, p-JNK, p-p38, pSmad3L, Smad4, Imp 7/8, PAI-1 (2) ↑ p38 ↓ p-ERK, p-JNK |
[ | 32 | ] | ||
| Multiple myeloma and myeloid leukemia | 99.9% ethanol | U266, U937 | 25, 50, 100, 200 µg/mL; 24 h | Induction of apoptosis | ↑ miR-216b, p-ATF4, p-eIf2, p-PERK, ROS, CHOP, c-PARP, c-caspase-3 ↓ c-Jun |
[ | 33 | ] | ||
| Non-small cell lung cancer (NSCLC) | Methanol extract | (CTN-compounds of tanshinone) | (1) Glc-82 (2) BALB/c mice |
(1) 20, 40 µg/mL; 24 h (2) 40 mg; 22 days |
Induction of apoptosis | ↑ p53, p21, c-caspase-3, -9, c-PARP1, PTEN, Bax ↓ Bcl-2, Bcl-xl, p-Akt |
[ | 34 | ] | |
| Non-small cell lung cancer (NSCLC) | Oldenlandia diffusa, Salvia miltiorrhiza 50% EtOH extract (5:2) | (1) A549, H460 (2) HUVECs (3) H460 xenograft model |
(1,2) PR 2.5 µg/mL + OS 180 µg/mL; 24 h (3) PR 125 µg/kg + OS 20 mg/kg; 18 days |
Antiangiogenic and apoptotic effects | ↑ c-caspase-3 ↓ p-STAT3, pro-PARP, Bcl-2, cyclin E, cyclin A, CDK2, E2F1, p-ERK, p-Akt, COX-2, SOCS-1, p-Src, VEGF, p-VEGFR2 |
[ | 35 | ] | ||
| Oral cancer | Double-distilled water, 95% ethanol or 1:1 water/ethanol | (1) HSC-3, OC-2 (2) BALB/cNU mice |
(1) 10, 25, 50 µg/mL; 48, 72 h (2) 50, 100 mg/kg; 34 days |
Inhibition of oral squamous carcinoma cell proliferation | ↑ c-caspase-3 ↓ XIAP, survivin |
[ | 36 | ] | ||
| Oral cancer | 95% ethanol | (1) SAS, SCC25, Oec-ml (2) KB, KB7D, KB tax, KB100, KB Vin, KB Vin 10 (3) SAS xenograft animal model |
(1) 0.625, 1.25, 2.5, 5, 10, 20, 30 µg/mL; 24 h (2) 2.5, 5, 10, 20, 40, 80 µg/mL; 24 h (3) 10 mg/kg; 32 days |
Inhibition of proliferation of oral cancer cell | ↑ c-caspase-3 ↓ XIAP |
[ | 37 | ] | ||
| Prostate cancer | Acetonitrile | (1) PC-3 (2) PC-3 xenograft mouse model |
(1) 20 µg/mL; 24, 48, 72 h (2) 100 mg/kg; 6 weeks |
Inhibitory effect on the growth of prostate cancer cell | ↑ ROS, c-caspase-3, -9, c-PARP, p21 ↓ Bcl-2, CDK2, CDK4, cyclin D1 |
[ | 38 | ] | ||
| Various cancers | 100% ethanol or 100% acetone | AGS, A549, HCT116, LNCaP, MCF7 | 5, 10, 20, 40 µg/mL; 24 h | Inhibitory effect on the growth of cancer cells | [ | 39 | ] | |||
| Multidrug-resistant cancer | Dichloromethane-methanol (1:1) | CCRF-CEM | (1) 3, 10, 30 µg/mL; 1 h (2) 5, 10, 20, 40 µg/mL; N/A (3) 20 µg/mL; 24, 2 h |
Cytotoxicity towards multidrug-resistant cancer cells | ↑ ROS, p-JNK, p-ERK1/2, p-p38, c-caspase-3, -7, -9, c-PARP ↓ p65 translocation |
[ | 40 | ] |
MMP-9, matrix metalloproteinase-9; MAPK, mitogen-activated protein kinase; AP-1, activator protein-1; SD, Sprague Dawley; TGF-β, transforming growth factor-β; miR-145, microRNA-145; miR-21, microRNA-21; p-ERK, phospho-extracellular-signal-regulated-kinase; p-JNK, phospho-c-Jun N terminal kinase; p-p38, phosho-p38; HSCs, hepatic stellate cells; Imp, importins; PAI-1, plasminogen activator inhibitor 1; p-ATF4, phospho-activating transcription factor 4; p-eIF2, phospho-eukaryotic initiation factor 2; p-PERK, phospho-protein kinase RNA-like endoplasmic reticulum kinase; CHOP, CCAAT-enhancer-binding protein homologous protein; c-PARP, cleaved poly ADP-ribose polymerase; c-caspase-3, cleaved caspase-3; CTN, compounds of tanshinone; PTEN, phosphatase and tensin homolog deleted on chromosome ten; Bax, Bcl-2 associated X-protein; Bcl-2, B-cell lymphoma 2; Bcl-xl, B-cell lymphoma-extra large; p-Akt, phospho-Akt (protein kinase B); HUVEC, human umbilical vein endothelial cell; PR, tetraarsenic hexoxide; OS, Olendlandia diffusa and Salvia miltiorrhiza extract; p-STAT3, phospho-signal transducer and activator of transcription 3 (Tyr705); VEGF, vascular endothelial growth factor; CDK, cyclin-dependent kinase; XIAP, X-linked inhibitor of apoptosis protein; ROS, reactive oxygen species.
