You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Ramesh L. Gardas.
Deep eutectic solvent (DES) are a highly non-ideal mixture of two biodegradable components (HBA and HBD) associated with strong hydrogen bonding interactions.
- deep eutectic solvents
- physicochemical properties
- applications
1. Definition
Generally, deep eutectic solvent (DES) are a highly non-ideal mixture of two biodegradable components (HBA and HBD) associated with strong hydrogen bonding interactions. The resulting liquid will have a melting point lower than the melting points of both HBA and HBD. They are broadly defined as liquid produced due to greater depression in the freezing point. Most DESs are a combination of quaternary ammonium salt, as HBA and HBD have the ability to form hydrogen bonding with HBA [2][1]. DESs were first introduced in 2003 by Abbott et al. for the mixture of ChCl (HBA) and urea (HBD) [17][2]. They showed that clear, deep eutectic liquid formed when ChCl with a melting point of 302 °C and urea with a melting point of 133 °C are mixed together in a definite molar ratio. The melting points of DESs were found to decrease to very low levels, around 12 °C, which is lower than the melting point of the two mixing compounds. According to this definition, many DESs have been prepared and applied as a solvent in organic reactions, extraction of dyes, protein, nucleic acids, metals, azeotropic separation, and more [18,19,20,21][3][4][5][6]. The list of HBAs and HBDs commonly used to prepare DESs is listed in Figure 1. The SciFinder analysis dated 31st December 2021 displayed 7226 publications for the term “Deep Eutectic Solvents”. It is evident from Figure 1 that the maximum publications were reported in 2021, and research in the subject of DESs is continuously growing.
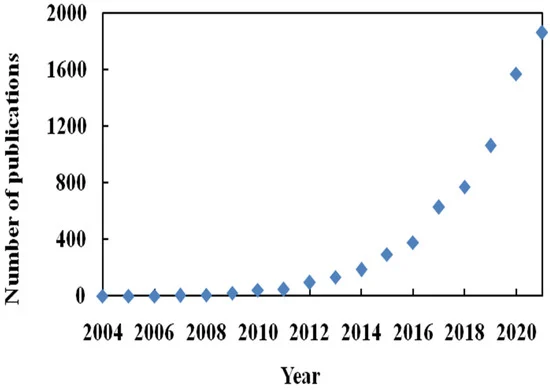
Figure 1. Number of publications per year for term deep eutectic solvents.
2. Method of Preparation
As discussed in the introduction, DESs are prepared by 100% atom economy route, as it involves simple mixing of HBA and HBD, and all other steps such as purification and waste disposal were eliminated or not required. (i) The most common preparation method, HBA and HBD of DESs, were heated and continuously stirred together in an inert atmosphere until homogeneous liquids were formed [17][2]. (ii) In the second approach, the evaporating method, the DES components were first dissolved in water. The water was then evaporated under vacuum at 323 K. The resultant mixture was kept in the desiccator until the attainment of stable weight. (iii) In the grinding method, solid HBA and HBD were added to a mortar kept in a glove box under an inert nitrogen atmosphere and ground continuously until a homogeneous transparent liquid was formed [25][7]. (iv) In the freeze-drying technique, HBA and HBD were first dissolved in water (5 wt%). The two aqueous solutions are mixed and then frozen. After that, the mixture was kept freeze-dried to obtain homogeneous and clear liquid [26][8].
2.3. Types of DES
2.1. Types of Deep Eutectic Solvent (DES)
DESs were presented using the general formula: R+A−xB, where R+ is ammonium, sulfonium, and phosphonium cation core. A and B are Lewis base with halide anion and Levis acid, respectively [20][5]. The complex formation is observed between x and Lewis or Brønsted acid B (x defined as B number of molecules reacted with anion). DESs are mainly classified based upon the nature of the HBD used, as shown in Table 1. Four main types of DESs have been reported. The possibility of a fifth type of DES has also been reported, but not enough literature about it is available yet.
Table 1. Classification of DESs.
| Type of DES | General Formula | Terms | Example | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | R | + | A | − | + cMCl | x | M = In, Zn, Fe, Al, Sn | ChCl + SnCl | 2 | ||||||
| II | R | + | A | − | + cMCl | x | .cH | 2 | O | M = Ni, Cr, Fe, Cu | ChCl + FeCl | 3 | ·6H | 2 | O |
| III | R | + | A | − | + cRW | W = OH, CONH | 2, | COOH | ChCl + Urea | ||||||
| IV | MCl | x | + cRW | M = Al, Zn and W = CONH | 2 | , OH | ZnCl | 2 | + Urea | ||||||
| V | HBD + HBA | HBD = hydrogen bond donor HBA = hydrogen bond acceptor |
Thymol + Menthol |
Type I DESs: This type of DES can be prepared from quaternary ammonium salt and metal chloride. Type I DESs formed with imidazolium salts and various metal halides such as ZnCl2, FeCl2, AgCl, CuCl2, CdCl2, LiCl, SnCl2, and SnCl4 [27,28][9][10]. The low melting point non-hydrated metal halides used to form type I DESs are very few; hence, fewer HBD combinations are available for this type of DES.
Type II DESs: This type of DES can be composed of quaternary ammonium salt and hydrate of metal chloride hydrate [29][11].
Type III DESs: This type of DES can be prepared using quaternary ammonium salt as HBA and HBD. This type of DES is widely studied. These DESs are mainly composed of choline chloride and HBDs (carboxylic acids, alcohols, amides, and carbohydrates, etc.) The HBA used in the preparation of this kind of DES are listed in Figure 2. These types of DESs are the most important due to their ability to solvate a wide range of transition metal species [17][2]. These solvents are simple to prepare, less expensive, relatively unreactive with water, and many are biodegradable. A wide range of HBDs (Figure 3) are available for the preparation of this class of DES. The physical properties of this type of DES are dependent on the nature of the hydrogen bond donor and can be customized easily for any given application.
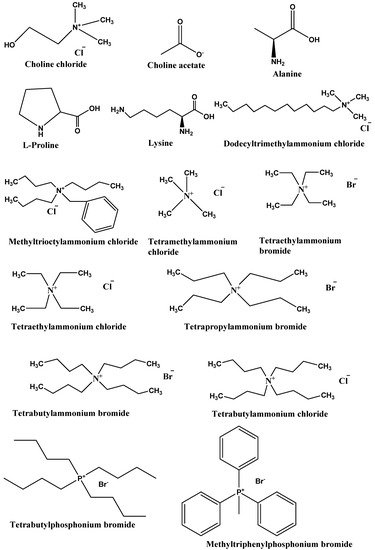
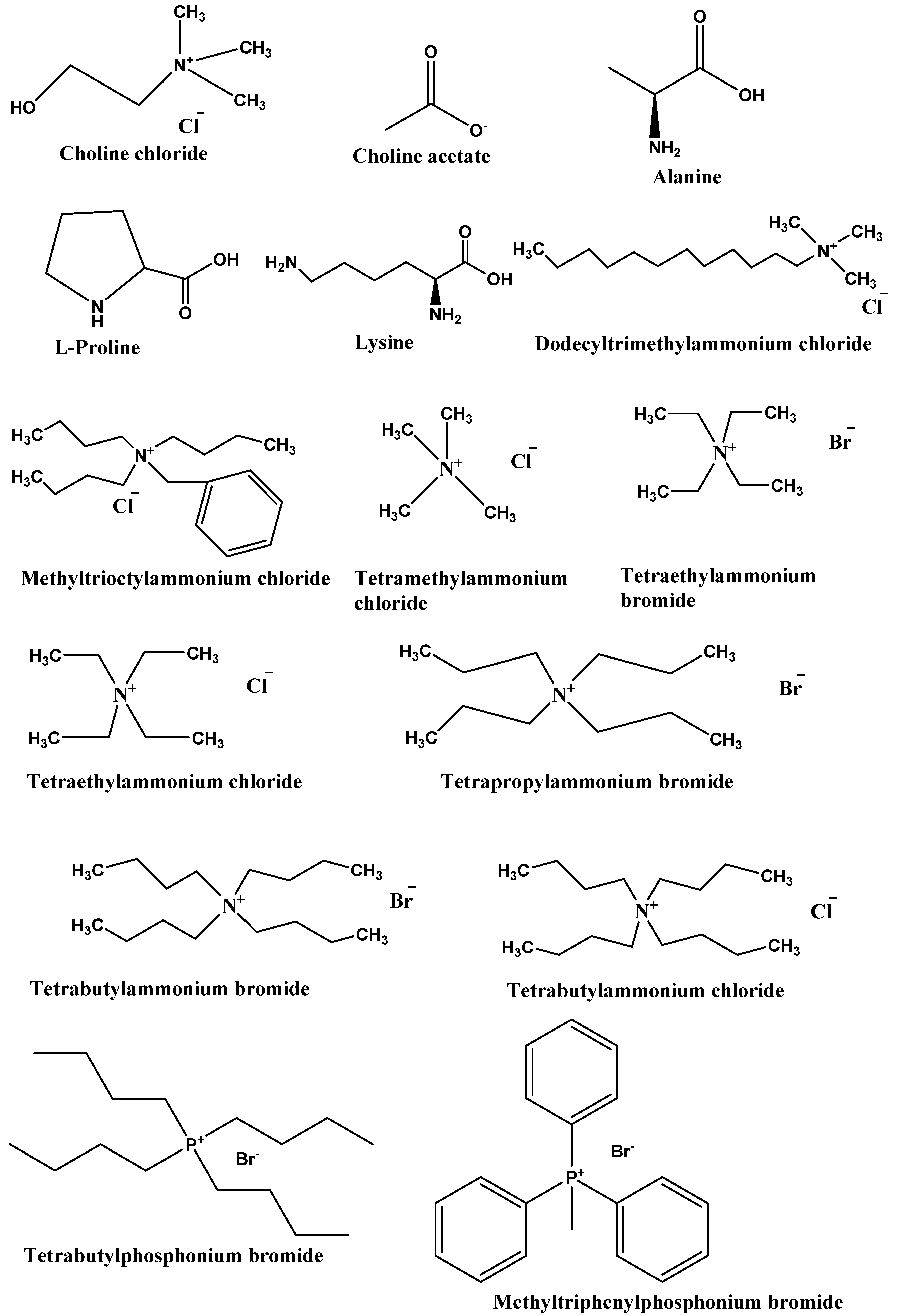 Figure 2. List of commonly used HBAs in preparation of DESs.
Figure 2. List of commonly used HBAs in preparation of DESs.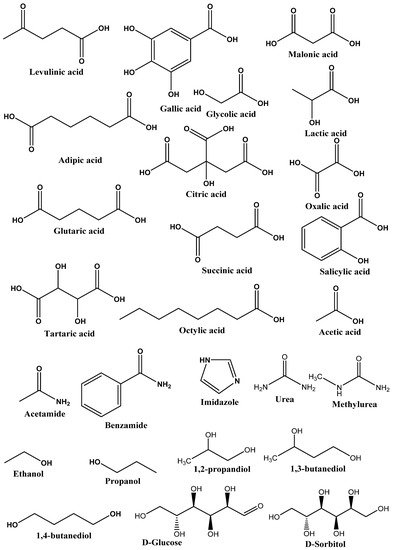
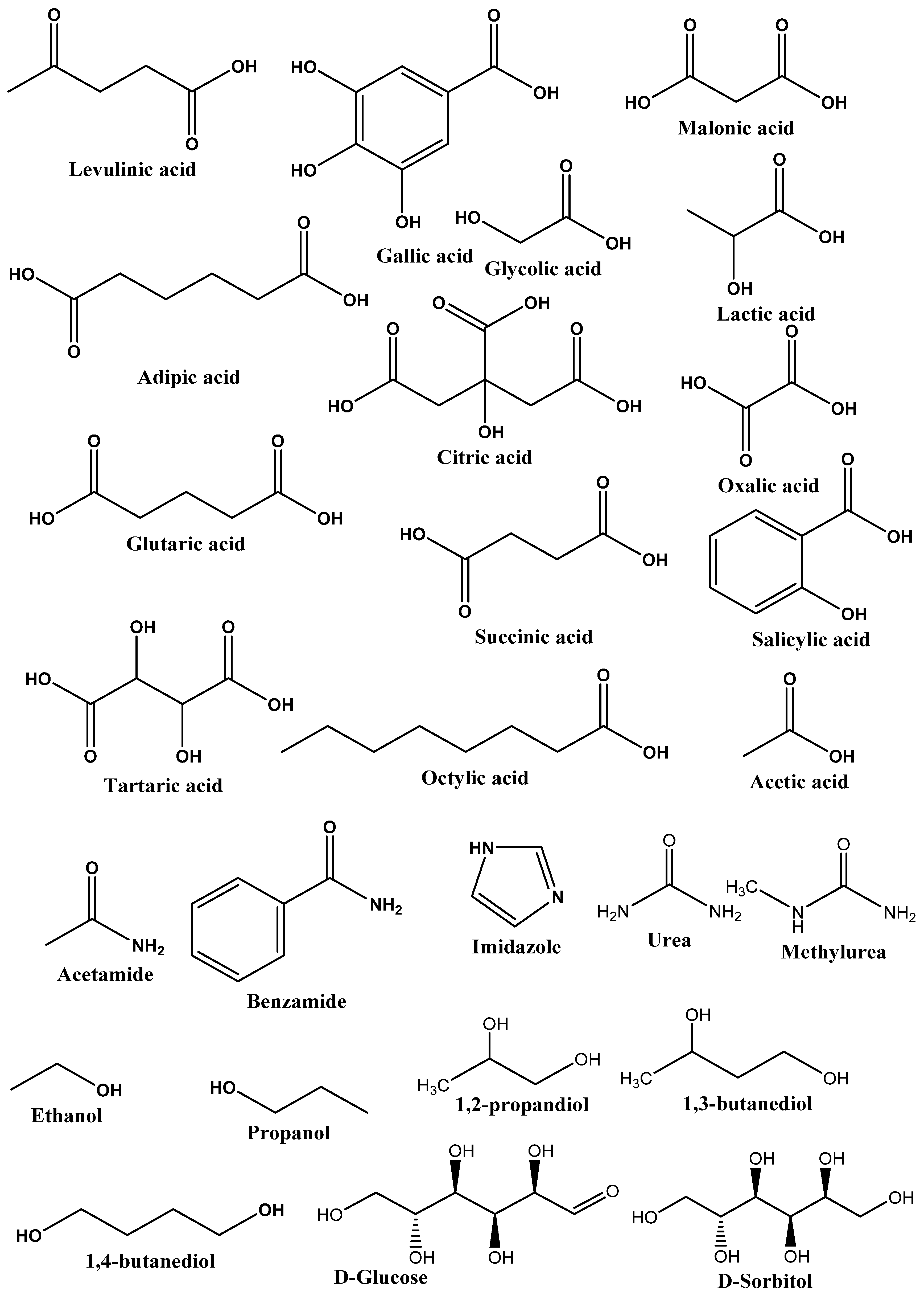 Figure 3. List of commonly used HBDs in preparation of DESs.
Figure 3. List of commonly used HBDs in preparation of DESs.Type IV DESs: These types of DESs are composed of metal chloride (particularly transition metal chloride) and HBD. It was reported to form a DES using a mixture of ZnCl2 and urea. In non-aqueous solvents, these metal salts usually do not ionize but form a DES with ethylene glycol, acetamide, and 1,6-hexanediol [29,30][11][12].
Type V DESs: These types of DESs are a relatively new class mixture of non-ionic molecular HBA and HBD [31][13]. Although it exhibits depression in melting points like DESs, there is no ionic contribution. Hydrogen bonding was especially predominant in this class of DESs. There is a possibility of another new class of mixture that does not fit precisely into this category. However, they exhibit deep depression in melting points like a mixture of Brønsted or Lowry acids:bases.
3. Applications of DES
3.1. Drug Delivery/Solubilization
The drug discovery and drug delivery of new drugs often face challenges such as safety, efficacy, cost, and availability [84,85,86,87,88][14][15][16][17][18]. To overcome this, existing drugs are now modified to improve their formulation or drug conversion (salts/ester), to introduce new combinations of existing drugs, or to change the route of drug administration [84,85][14][15]. The transport and processing of pharmaceuticals requires a solvent, and this purpose is fulfilled using water, unless the drug is a hydrophobic drug that is poorly soluble or insoluble [85][15]. Due to low permeation and bioavailability, the low solubility of available or developing drugs influences the therapeutic action [85][15]. For the oral drug delivery system, the improvement of drug bioavailability and solubility limits the drug delivery/administration [85][15]. One of the strategies is to have an improved formulation, with active pharmaceutical ingredient (API) being encapsulated, dispersed, or loaded inside a drug carrier [85,86][15][16]. One such study is the absorption of sulfathiazole from a eutectic mixture with urea compared to the absorption of ordinary sulfathiazole [87][17]. Thus, various studies [81,82,83][19][20][21] are conducted on deep eutectic solvent (DES) and eutectic mixture to improve the API solubility and dissolution behavior.
The approved drugs or drugs under development are poorly water-soluble, so one of the characteristics to improve the drug efficiency, permeability, and bioavailability is enhancing drug hydrophilicity [85,88][15][18]. For instance, manipulating drug formulation may increase the solubility and dissolution rate of BCS class II (Biopharmaceutics Classification System II) substances in gastrointestinal fluids, increasing bioavailability [85,89][15][22]. The efficiency of poorly water-soluble drugs can be improved by modifying API dosage, novel drug administration routes, and adopting a suitable combination of active ingredients [85,88][15][18]. One strategy is to form API dispersion inside a biocompatible polymer matrix or search for alternative solvents [81,84][19][14]. In this regard, ionic liquids have been utilized as a suitable solvent system for API, owing to the unique physicochemical properties of IL [85,86,87,88][15][16][17][18]. A suitable cation–anion combination can be made to synthesize numerous ILs [85][15] with appropriate physical properties desirable for the dissolution/loading of APIs [85][15]. However, ILs still suffer from biodegradability or toxicity limitations [85][15]. So, a search for a new biocompatible solvent system with negligible toxicity for API dissolution or solubility is required to improve and develop drug formulations [85,88][15][18].
References
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents Angew. Chem. Int. Ed. 2013, 52, 3074–3075.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71.
- Carriazo, D.; Serrano, M.C.; Gutierrez, M.C.; Ferrer, M.L.; Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014.
- Pena-Pereira, F.; Namiesnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2015, 7, 1–18.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuel. 2015, 29, 2616–2644.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids, ACS Sustain. Chem. Eng. 2014, 2, 2416–2425.
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures. Inorg. Chem. 2004, 43, 3447.
- Xu, W.G.; Lu, X.M.; Zhang, Q.G.; Gui, J.S.; Yang, J.Z. study on thermodynamic properties of ionic liquids BMIMGaCl2. Chin. J. Chem. 2006, 24, 331.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic Liquid Analogues Formed from Hydrated Metal Salts. Chem. Eur. J. 2004, 10, 3769.
- Gambino, M.; Bros, J.P. Heat capacity of urea and of a group of eutectic mixtures based on urea between 30 and 140 °C. Thermochim. Acta 1988, 127, 223–236.
- Abranches, D.O.; Martins, M.A.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic Hydrogen Bond Donors in the Formation of Non-ionic Deep Eutectic Solvents: The Quest for Type V DES. Chem. Commun. 2019, 55, 10253–10256.
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453.
- Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Del. 2019, 16, 497–506.
- Indoria, S.; Singh, V.; Hsieh, M.F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314.
- Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872.
- Gutierrez, A.; Aparicio, S.; Atilhan, M. Design of arginine-based therapeutic deep eutectic solvents as drug solubilization vehicles for active pharmaceutical ingredients. Phys. Chem. Chem. Phys. 2019, 21, 10621–10634.
- Maugeri, Z.; Domınguez, P. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425.
- Agostino, C.; Harris, R.C.; Abbott, A.P.; Gladden, L.F.; Mantle, M.D. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391.
- Yusof, R.; Abdulmalek, E.; Sirat, K.; Basyaruddin, M.; Rahman, M.A. Tetrabutylammonium bromide (TBABr)-based deep eutectic solvents (DESs) and their physical properties. Molecules 2014, 19, 8011–8026.
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316.
More
