You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Malu Ram Yadav.
Heat stress (HS) is one of the major abiotic stresses affecting the production and quality of wheat. Rising temperatures are particularly threatening to wheat production.
- wheat
- climate change
- heat stress
1. Introduction
Wheat (Triticum aestivum L.) is one of the most widely cultivated cereal crops in the world, making a significant contribution to global cereal production (28%) and trade (41.5%) [1]. About 198 million tonnes of additional wheat grain will be required to feed the increasing human population predicted to be 9.8 billion by 2050 [2]. Wheat production is vulnerable to abiotic and biotic stresses with a stagnant or declining rates of productivity across the globe [3]. Different climatic factors interact spatiotemporally and influence crop growth and production. To better cope with the climatic variables (e.g., temperature and rainfall), their impacts must be quantified and understood. The adverse impact of heat stress (HS) induced through rising ambient temperature and unpredictable climatic variations is clear and threatening wheat production in all ecologies (temperate, subtropical and in tropical) [4,5,6][4][5][6].
The mean global temperature of the Earth is expected to increase by 1.5 °C within the next two decades [7]. Recent analysis from scientific communities, including the Goddard Institute for Space Studies (GISS), indicated an increase in average global temperature of 1.04 °C from 1880 to 2019 (NOAA, 2020). This elevated temperature is causing heat stress (HS) that triggers significant changes in the biological and developmental process of wheat, leading to a reduction in grain production [8,9][8][9] and grain quality [10]. The optimal temperature requirements of wheat at different growth stages are summarized in Table 1. Wheat is most susceptible to elevated temperature stress especially during anthesis stage and less likely to recover if stressed at this critical stage [11,12,13][11][12][13]. HS can affect the growth and development of wheat through alteration of physio-bio-chemical processes, such as photosynthesis, respiration, oxidative damage, activity of stress-induced hormones, proteins and anti-oxidative enzymes, water and nutrient relations, and yield forming attributes (biomass, tiller count, grain number and size) upon exposure to temperatures above the optimum range [14,15,16,17,18,19][14][15][16][17][18][19].
Table 1.
Optimal temperature requirements of wheat at different growth stages (Adopted from Khan et al. [20]).
| Stages | Optimum Temperature (°C) |
Minimum Temperature (°C) |
Maximum Temperature (°C) |
|---|---|---|---|
| Seed germination | 20–25 ± 1.2 | 3.5–5.5 ± 0.44 | 35 ± 1.02 |
| Root growth | 17.2 ± 0.87 |
29][30][31][32]. However, the limited progress and success in the development of HS resilience in wheat can be attributed to the lack of coordinated efforts by plant breeders, biotechnologists, agronomists, and physiologists. Therefore, the adoption of a holistic multidisciplinary approach integrating outcomes of breeding, physiological, biotechnological, and agronomical options is needed for providing practical solutions.
2. Responses of Wheat to HS
2.1. Morphological and Phenological Responses
HS affects diverse morpho-phenological stages, such as germination, seedling emergence, tillering, floral initiation, pollination, fertilization, and ultimately yield and grain quality [11,12,14][11][12][14] (Figure 1 and Table 2). However, the impact of HS on these phenological stages depends upon the magnitude and length of exposure, genotypes, soil moisture status, and concentration of elevated carbon in the atmosphere [21,33][21][33]. The HS at the early seedling stage leads to poor seedling establishment. At the early vegetative stage of the crop, HS reduces root and shoot growth reducing the green leaf area and the number of effective tillers per plant [34,35][34][35]. Prolonged exposure to elevated temperature leads to cell injuries and the gradual shedding of leaves and abortion of flower and fruits [35,36][35][36]. during and just after anthesis (within 10 days) leads to flower abortion and thus reducing grain number, grain filling and maturity period [11,37,38][11][37][38]. The HS during the initiation of flowering to early grain filling stages (terminal HS) is more severe, causing a drastic reduction in the dry matter accumulation and grain quality of wheat. Therefore, more emphasis should be placed upon the management of terminal HS to sustain the wheat yields [24,39][24][39].
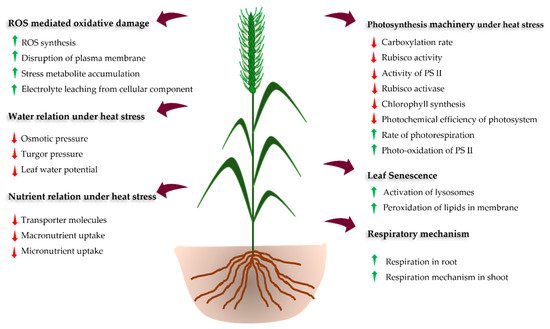
Figure 1.
Schematic diagram showing impacts and responses of plants to heat stress.
Table 2.
Impact of heat stress on different traits/biological processes of wheat.
| Trait/ Biological Process |
Responses/Consequences/Impact | References |
|---|
| Morpho-phenological behavior | Poor germination and seedling establishment | [34,40] | [34][40] |
| Reduction in root length, shoot growth and dry matter | [34,41] | [34][413.50 ± 0.73 | 24.0 ± 1.21 |
| Shoot growth | 18.5 ± 1.90 | 4.50 ± 0.76 | 20.1 ± 0.64 |
| Leaf initiation | 20.5 ± 1.25 | 1.50 ± 0.52 | 23.5 ± 0.95 |
| Terminal spikelet | 16.0 ± 2.30 | 2.50 ± 0.49 | 20.0 ± 1.60 |
| Anthesis | 23.0 ± 1.75 | 10.0 ± 1.12 | 26.0 ± 1.01 |
| Grain filling duration | 26.0 ± 1.53 | 13.0 ± 1.45 | 30.0 ± 2.13 |
HS impacts the wheat plant in both indirect and direct ways. Indirect injury includes poor seed germination, decreased growth, enhancement in leaf senescence, and a reduction in photosynthesis and floret fertility [12[12][13][21],13,21], whereas direct injuries include protein denaturation, increased fluidity of membrane lipids, and aggregation of proteins [18,19][18][19]. However, the consequence of HS depends on the duration and intensity of stress, or genotypes [2,21][2][21]. Therefore, emphasis should be placed on sustaining the wheat yields through the identification of tolerant genotypes and promotion of breeding strategies and management practices that can help to build HS resilience and safeguard the wheat production from HS [22]. An improved understanding of morphological and physiological traits associated with HS tolerance has pragmatic implications for devising countermeasures, e.g., to identify various tolerance mechanisms and their use in alleviation strategies [23,24,25][23][24][25]. The mapping of genomic regions governing such physiological traits helped to identify genes (or QTLs) conferring HS tolerance, which serve as a strong base for the marker-assisted (MAS) breeding of HS tolerance in wheat [24,26][24][26]. Conventional breeding approaches (screening and selection of germplasm) and molecular breeding are promising genetic strategies for the development of HS tolerant wheat genotypes and the introduction of these cultivars in the non-conventional area will help to overcome the issue of food and nutritional security [2,27][2][27].
Besides, different biotechnological tools, e.g., transgenics and gene editing, along with recently developed omics approaches can help to develop HS tolerant cultivars [28]. The most reliable and inexpensive method to improve plant resilience to HS includes a combination of stress tolerant genotypes and agronomic management strategies, such as the application of exogenous protectants, adoption of climate-smart cultivation practices including conservation agriculture (CA), micro-irrigation and mulching, and use of cultured soil microbes [6,29,30,31,32][6][
| ] |
| Reduction in effective tiller | |||
| [ | 42] | ||
| Reduced ear length, number of spikelet and fertile floret | [43,44] | [43][44] | |
| Abortion of flower and fruits | [11,12,41] | [11][12][41] | |
| Shedding of leaves | [45,46] | [45][46] | |
| Reduction in phenological duration of crop | [14,47] | [14][47] | |
| Reduced days to germination, anthesis and maturity | [14,43] | [14][43] | |
| Reduction in germination of pollen grains and spikelet fertility | [11,12,48,49] | [11][12][48][49] | |
| Reduced grain filling period | [11,14,43,45] | [11][14][43][45] | |
| Grain development and quality | Reduction in number or size of grain | [11,14,19] | [11][14][19] |
| Reduction in harvesting index | [48] | ||
| Increases rate of grain filling but shortened grain filling duration | [50] | ||
| Reduction in transportation of photo-assimilates to grain | [51] | ||
| Increase in grain protein and reduction in quality of proteins | [52] | ||
| Reduced starch synthesis | [51,52] | [51][52] | |
| Reduced total soluble sugar and super molecules | [51,53] | [51][53] | |
| Reduction in essential amino acids | [50] | ||
| Reduced bread making quality | [52] | ||
| Reduction in flour quality and sedimentation index | [50,54] | [50][54] | |
| Reduction in economic grain yield | [51,55] | [51][55] | |
| Physiological and growth behavior | Reduced photosynthesis and photosynthetic efficiency | [14,18,19,56,57] | [14][18][19][56][57] |
| Increase in respiration and photorespiration at mild heat stress | [56,57] | [56][57] | |
| Increase in leaf senescence and reduction of chlorophyll content | [14,18,19,39,46] | [14][18][19][39][46] | |
| Reduction in the relative water content and leaf water potential | [45,58] | [45][58] | |
| Increased transpiration and decreased stomatal conductance | [59] | ||
| Decrease uptake and translocation of water | [50,60] | [50][60] | |
| Increased canopy temperature | [61] | ||
| Reduction in uptake, assimilation, and translocation of nutrient | [62,63] | [62][63] | |
| Reduction in specific leaf weight, leaf width and total dry matter | [38,64] | [38][64] | |
| Molecular responses | Enhanced production of reactive oxygen species (ROS) | [18,19,65,66] | [18][19][65][66] |
| Higher accumulation of osmolytes | [36,65] | [36][65] | |
| Destruction of plasma, mitochondrial and chloroplast membrane | [34] | ||
| Reduction in Rubisco activity | [66] | ||
| Reduction in soluble and rubisco binding proteins | [16] | ||
| Denaturation and aggregation of seed proteins | [67] | ||
| Higher accumulation of heat shock proteins | [68,69] | [68][69] | |
| Activation of antioxidant system and associated molecules | [70,71] | [70][71] |
2.2. Physiological and Molecular Responses
Most of the physiological functions in plants are temperature dependent and any deviation over the optimum temperature hampers the growth and development of plants (Table 2). HS reduces photosynthetic efficiency which eventually affects the plant growth and biomass production [22,56][22][56]. The reduction in photosynthesis rate under HS is correlated with the increase in the non-photorespiratory processes and reduction of soluble proteins, Rubisco and Rubisco binding proteins [72,73][72][73]. These sequential changes results in reduced leaf area, less effective photosynthetic machinery, early leaf senescence, overproduction of reactive oxygen species (ROS), disruption of thylakoid membrane, alteration of enzyme action, and denaturation of heat shock proteins (HSPs), which ultimately reduces wheat productivity [6,55,74][6][55][74]. The reduction in total chlorophyll content with accelerated leaf senescence diminishes the photosynthetic capacity of the plant [61]. Furthermore, respiration and photorespiration rate increase with temperature above the threshold level, resulting in the reduction of availability and transport of photoassimilates from leaves to the grain. This hampers the growth and grain filling process [75,76][75][76].
Water status in the plant is crucial especially under HS as the temperature of plant tissue (canopy temperature) can be optimized by water uptake and transpiration. Thus, leaf relative water content (LRWC), leaf water potential (LWP), rate of transpiration, and stomatal conductance are strongly influenced by canopy temperature (Table 2) [59]. Higher vapor pressure deficits under HS lead to a higher evapotranspiration rate which ultimately decreases LWRC and LWP (Table 2). However, limited information is available about the effect of HS in relation to the nutrient status of the crop [77]. The uptake and translocation of nitrogen (N) in wheat declined under HS due to a reduction in nitrate reductase activity (NRA) in plants [62]. Phosphorus (P), potassium (K), sulphur (S), and sodium (Na) helps to maintain the redox state of the cell and hence protects the cell membranes, improving the antioxidant defense system and osmotic potential, which ultimately enhances the photosynthetic rate. These nutrients reduce the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and retain the photosynthetic electron transport activity, which helps to reduce ROS thus maintain the redox state of the cell under HS. However, reduced root growth under HS reduces uptake, assimilation, and translocation of most of the nutrients [78].
HS triggers the production of ROS, such as super-oxides (O2−), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2), resulting in severe oxidative damage to the plant [79]. HS can also damage the plasma, mitochondrial, and chloroplast membranes due to lipid peroxidation and concomitant H2O2 production [80]. To the eliminate the accumulated ROS and maintain metabolic activities and productivity [81], the plant has a well-organized cellular stress-response system (defense systems) composed mainly of transcription factors (TFs) and HSPs [82]. Plants possess signal transduction molecules (Calcium-dependent protein kinases (CDPKs), mitogen-activated protein kinase (MAPKs), sugar (as signalling molecule), and phytohormones) that act as an activator of the stress-responsive gene during HS [68,83][68][83]. For example, dehydration-responsive element-binding protein (DREB) is a signal transduction molecule that activates the stress-responsive genes through stress signaling induced expression in wheat. These signal transduction molecules together with TFs activate stress-responsive genes [84,85][84][85]. For example, TaHSFA6f gene has been reported to function in sensing the HS and overexpression of this gene imparted heat tolerance in wheat [86].
2.3. Heat Stress and Sensitive Stages of Wheat
The impact of HS is more severe during reproductive stages in comparison to the vegetative phase [11,12,52][11][12][52]. HS hinders pollen tube growth, resulting in reduced germination of pollen grains on stigma and impacts embryo formation [12]. Translocation of assimilates and the rate and duration of grain filling are also directly affected by elevated temperature [87]. Exposure to high temperature stress during the division of pollen mother cells can reduce grain setting [11,12,88][11][12][88]. High temperature (3038 °C) during the reproductive phase can lead to the reduction of the total biomass of wheat up to 44% [48]. HS during the early grain filling stage declines the synthesis of starch in the wheat grain but increases the concentration of total soluble sugars (Table 2) [89]. Although the protein content increases under HS, the functionality of proteins significantly decreases, adversely affecting its end-use [90]. Even after a high percentage of grain protein, total protein yield reduces under HS due to a drastic reduction in the grain yield [91]. However, an increased level of protein content has been reported to reduce the sedimentation index, which affects the bread-making quality of wheat flour [92,93][92][93]. The heat-inducible accumulation of gliadins under HS also deteriorates the flour quality of wheat [94].
Temperature is the most important factor influencing the growth and development of wheat (Table 1). The growth and development start with seed germination, which requires optimum temperature and moisture. HS is an important key abiotic stress that severely affects the seedling establishment of wheat [95,96][95][96]. The germination index and germination potential of two wheat cultivars, namely DBA Aurora and L6, were significantly reduced under HS conditions [97]. The reduction in the germination percentage and seedling establishment under heat stress conditions might be due to a reduction in the antioxidant system, production of ROS, increased lipid peroxidation, and differential expression of miRNAs [16]. Recent studies on seedling establishment have revealed that the expression of miRNAs and epigenetic changes in wheat seedlings might be key factors that help to optimize the seed vigor for superior breeding of germplasm and varieties under heat stress [98]. The seedling with high vigor is projected to be more effective in radiation and resource use efficiency. High seedling vigor has no transgenerational effect on the vegetative and reproductive phase [99]. During the vegetative growth of wheat, the tissues exposed to HS leak electrolytes, damaging leaf members, and decreasing photosynthesis [100,101][100][101]. HS severely impacts on photosystem II and thylakoid membranes, which are most sensitive cell structures [102].
HS stress is a severe threat to wheat production when it occurs during reproductive and grain-filling phases (Table 2) [11,12,13,14,55][11][12][13][14][55]. Wheat crops experience HS in different phenological stages. However, the impact of HS during the reproductive phase is relatively more detrimental as compared to the vegetative phase which ultimately affects the grain quality and yield (Table 2). HS during reproductive stages of crop development decreases grain yield up to 30% [11,19,103][11][19][103]. The optimum temperature for anthesis and grain filling stage was suggested to be in the range of 12–24 °C, and 8–6 days before anthesis stages are identified to be the most sensitive stages to HS [11,19][11][19]. Likewise, results of 30 wheat crop models from different locations around the globe where mean temperatures in the growing season ranged from 15 to 32 °C with artificial heating indicated that grain yield of wheat started to decline at a majority of the wheat-growing locations [104]. The simulated median temperature impact on declining wheat yield ranged between 1% and 28% for an increase in temperature of 2 °C, and this value rose to between 6% and 55% for a temperature of 4 °C [105].
Moreover, the reduction in the number of spikelets per panicle, hundred-grain weight, and seed setting rate was reported in wheat under HS [2,12,19,106][2][12][19][106] (Table 2). Pollen tube development after pollination is extremely sensitive to heat stress, leading to abnormal ovary development, ultimately resulting in lower yield as well as quality of grains [104,107,108][104][107][108]. Harvest index (HI) and its correlation with heat stress is most significant in wheat among all cereals. HI plummets when daily mean temperatures cross 16 °C and a significant loss of yield occurs when it crosses 30 °C [12]. The impact is more severe when there is higher night temperature, resulting in reduced grain yield, attributed primarily to the reduced spike number [14,109,110][14][109][110]. HS induced through high night temperatures, especially during the grain filling stage, can reduce grain yield, HI, 1000-grain weight, and grain weight per spike [111]. These studies provide key traits for wheat breeders to enhance high night temperature tolerance in wheat.
References
- FAO. Quarterly Global Report No. 1; Rome FAO: Rome, Italy, 2020.
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37.
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317.
- Iqbal, M.; Raja, N.I.; Yasmeen, F.; Hussain, M.; Ejaz, M.; Shah, M.A. Impacts of Heat Stress on Wheat: A Critical Review. Adv. Crop Sci. Technol. 2017, 5, 1–9.
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; Volume 9781107057, pp. 1029–1136. ISBN 9781107415324.
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and molecular insights on wheat responses to heat stress. Plant. Cell Rep. 2021, 1, 1–18.
- IPCC; Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021.
- Fleitas, M.C.; Mondal, S.; Gerard, G.S.; Hernández-Espinosa, N.; Singh, R.P.; Crossa, J.; Guzmán, C. Identification of CIMMYT spring bread wheat germplasm maintaining superior grain yield and quality under heat-stress. J. Cereal Sci. 2020, 93, 102981.
- Pequeno, D.N.L.; Hernández-Ochoa, I.M.; Reynolds, M.; Sonder, K.; Moleromilan, A.; Robertson, R.D.; Lopes, M.S.; Xiong, W.; Kropff, M.; Asseng, S. Climate impact and adaptation to heat and drought stress of regional and global wheat production. Environ. Res. Lett. 2021, 16, 054070.
- Kumar, A.; Dash, G.K.; Barik, M.; Panda, P.A.; Lal, M.K.; Baig, M.J.; Swain, P. Effect of Drought stress on Resistant starch content and Glycemic index of rice (Oryza sativa L.). Starch/Staerke 2020, 72, 1900229.
- Prasad, P.V.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant. Biol. 2014, 41, 1261–1269.
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121.
- Aiqing, S.; Somayanda, I.; Sebastian, S.V.; Singh, K.; Gill, K.; Prasad, P.V.V.; Jagadish, S.V.K. Heat stress during flowering affects time of day of flowering, seed set, and grain quality in spring wheat. Crop Sci. 2018, 58, 380–392.
- Prasad, P.V.V.; Pisipati, S.R.; Ristic, Z.; Bukovnik, U.; Fritz, A.K. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Sci. 2008, 48, 2372–2380.
- Ullah, A.; Nadeem, F.; Nawaz, A.; Siddique, K.H.; Farooq, M. Heat stress effects on the reproductive physiology and yield of wheat. J. Agron. Crop Sci. 2022, 208, 1–17.
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684.
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592.
- Djanaguiraman, M.; Boyle, D.L.; Welti, R.; Jagadish, S.V.K.; Prasad, P.V.V. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 18, 1–17.
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020, 20, 268.
- Khan, A.; Ahmad, M.; Ahmed, M.; Iftikhar Hussain, M. Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants 2021, 10, 43.
- Kumar, R.R.; Ahuja, S.; Rai, G.K.; Kumar, S.; Mishra, D.; Kumar, S.N.; Rai, A.; Singh, B.; Chinnusamy, V.; Praveen, S. Silicon triggers the signalling molecules and stress-associated genes for alleviating the adverse effect of terminal heat stress in wheat with improved grain quality. Acta Physiol. Plant. 2022, 44, 30.
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069.
- Langridge, P.; Reynolds, M. Breeding for drought and heat tolerance in wheat. Theor. Appl. Genet. 2021, 134, 1753–1769.
- Rehman, H.U.; Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.M.A.; Reynolds, M. Evaluation of physiological and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants 2021, 10, 455.
- Zhai, H.; Jiang, C.; Zhao, Y.; Yang, S.; Li, Y.; Yan, K.; Wu, S.; Luo, B.; Du, Y.; Jin, H.; et al. Wheat heat tolerance is impaired by heightened deletions in the distal end of 4AL chromosomal arm. Plant Biotechnol. J. 2021, 19, 1038–1051.
- Kaushal, N.; Bhandari, K.; Siddique, K.H.M.; Nayyar, H. Food crops face rising temperatures: An overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food Agric. 2016, 2, 1134380.
- Liu, Z.; Qin, J.; Tian, X.; Xu, S.; Wang, Y.; Li, H.; Wang, X.; Peng, H.; Yao, Y.; Hu, Z.; et al. Global profiling of alternative splicing landscape responsive to drought, heat and their combination in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2018, 16, 714–726.
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43.
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 34011.
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Kumar, A.; Tiwari, R.K.; Lal, M.K.; Singh, B. An Insight into Microbes Mediated Heavy Metal Detoxification in Plants: A Review. J. Soil Sci. Plant Nutr. 2021, 41, 1–23.
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 172, 1212–1226.
- Kumar, A.; Singh, B.; Raigond, P.; Sahu, C.; Mishra, U.N.; Sharma, S.; Lal, M.K. Phytic acid: Blessing in disguise, a prime compound required for both plant and human nutrition. Food Res. Int. 2021, 142, 110193.
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163.
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842.
- Tewolde, H.; Fernandez, C.J.; Erickson, C.A. Wheat cultivars adapted to post-heading high temperature stress. J. Agron. Crop Sci. 2006, 192, 111–120.
- Rodríguez, M.; Canales, E.; Borrás-Hidalgo, O. Molecular aspects of abiotic stress in plants. Biotecnol. Apl. 2005, 22, 1–10.
- Bheemanahalli, R.; Sunoj, V.S.J.; Saripalli, G.; Prasad, P.V.V.; Balyan, H.S.; Gupta, P.K.; Grant, N.; Gill, K.S.; Jagadish, S.V.K. Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci. 2019, 59, 684–696.
- Mendanha, T.; Rosenqvist, E.; Hyldgaard, B.; Ottosen, C.O. Heat priming effects on anthesis heat stress in wheat cultivars (Triticum aestivum L.) with contrasting tolerance to heat stress. Plant Physiol. Biochem. 2018, 132, 213–221.
- Kumar, R.R.; Tasleem, M.; Jain, M.; Ahuja, S.; Goswami, S.; Bakshi, S.; Jambhulkar, S.; Singh, S.D.; Singh, G.P.; Pathak, H.; et al. Nitric oxide triggered defense network in wheat: Augmenting tolerance and grain-quality related traits under heat-induced oxidative damage. Environ. Exp. Bot. 2019, 158, 189–204.
- Nahar, K.; Ahamed, K.U.; Fujita, M. Phenological Variation and its Relation with Yield in several Wheat (Triticum aestivum L.) Cultivars under Normal and Late Sowing Mediated Heat Stress Condition. Not. Sci. Biol. 2010, 2, 51–56.
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Combined effects of elevated and high night temperature on carbon assimilation, nitrogen absorption, and the allocations of C and N by rice (Oryza sativa L.). Agric. For. Meteorol. 2010, 150, 1174–1181.
- Djanaguiraman, M.; Prasad, P.V.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007.
- Prasad, P.V.V.; Pisipati, S.R.; Momčilović, I.; Ristic, Z. Independent and Combined Effects of High Temperature and Drought Stress During Grain Filling on Plant Yield and Chloroplast EF-Tu Expression in Spring Wheat. J. Agron. Crop Sci. 2011, 197, 430–441.
- Matsui, T.; Omasa, K.; Horie, T. The difference in sterility due to high temperatures during the flowering period among Japonica-rice varieties. Plant Prod. Sci. 2001, 4, 90–93.
- Machado, S.; Paulsen, G.M. Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil 2001, 233, 179–187.
- Shah, N.H.; Paulsen, G.M. Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 2003, 257, 219–226.
- Viswanathan, C.; Khanna-Chopra, R. Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat (Triticum aestivum L.) varieties differing in grain weight stability. J. Agron. Crop Sci. 2001, 186, 1–7.
- Tahir, I.S.A.; Nakata, N. Remobilization of nitrogen and carbohydrate from stems of bread wheat in response to heat stress during grain filling. J. Agron. Crop Sci. 2005, 191, 106–115.
- Eltayeb, A.E.; Yamamoto, S.; Habora, M.E.E.; Yin, L.; Tsujimoto, H.; Tanaka, K. Transgenic potato overexpressing Arabidopsis cytosolic AtDHAR1 showed higher tolerance to herbicide, drought and salt stresses. Breed. Sci. 2011, 61, 3–10.
- Dias, N.S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Jerusalem artichoke (Helianthus tuberosus, L.) maintains high inulin, tuber yield, and antioxidant capacity under moderately-saline irrigation waters. Ind. Crops Prod. 2016, 94, 1009–1024.
- Asthir, B.; Bhatia, S. In vivo studies on artificial induction of thermotolerance to detached panicles of wheat (Triticum aestivum L) cultivars under heat stress. J. Food Sci. Technol. 2014, 51, 118–123.
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 871, 1–19.
- Lal, M.K.; Singh, B.; Sharma, S.; Singh, M.P.; Kumar, A. Glycemic index of starchy crops and factors affecting its digestibility: A review. Trends Food Sci. Technol. 2021, 111, 741–755.
- Wardlaw, I.F. Tansley Review No. 27 The control of carbon partitioning in plants. New Phytol. 1990, 116, 341–381.
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228.
- Cao, Z.; Yao, X.; Liu, H.; Liu, B.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Comparison of the abilities of vegetation indices and photosynthetic parameters to detect heat stress in wheat. Agric. For. Meteorol. 2019, 265, 121–136.
- Larkindale, J.; Mishkind, M.; Vierling, E. Plant Responses to High Temperature. In Plant Abiotic Stress; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 100–144. ISBN 1405122382.
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management; Springer: New York, NY, USA, 2009; Volume 29, pp. 185–212.
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. CRC Crit. Rev. Plant Sci. 2011, 30, 491–507.
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126.
- Shah, N.H.; Paulsen, G.M. Injury to Photosynthesis and Productivity from Interaction Between High Temperature and Drought During Maturation of Wheat. Asian J. Plant Sci. 2004, 4, 67–74.
- Klimenko, S.B.; Peshkova, A.A.; Dorofeev, N.V. Nitrate Reductase Activity During Heat Shock in Winter Wheat. J. Stress Physiol. Biochem. 2006, 2, 50–55.
- Paliwal, R.; Röder, M.S.; Kumar, U.; Srivastava, J.P.; Joshi, A.K. QTL mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor. Appl. Genet. 2012, 125, 561–575.
- Gupta, U.; Solanki, H. Impact of boron deficiency on plant growth. Int. J. Bioassays 2013, 2, 1048–1050.
- Kumar, R.R.; Singh, K.; Ahuja, S.; Tasleem, M.; Singh, I.; Kumar, S.; Grover, M.; Mishra, D.; Rai, G.K.; Goswami, S.; et al. Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct. Integr. Genom. 2019, 19, 329–348.
- Galmés, J.; Flexas, J.; Keys, A.J.; Cifre, J.; Mitchell, R.A.C.; Madgwick, P.J.; Haslam, R.P.; Medrano, H.; Parry, M.A.J. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ. 2005, 28, 571–579.
- López-Barón, N.; Gu, Y.; Vasanthan, T.; Hoover, R. Plant proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2017, 69, 19–27.
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007, 8, 125.
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266.
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51.
- Roy, S.; Arora, A.; Chinnusamy, V.; Singh, V.P. Endogenous reduced ascorbate: An indicator of plant water deficit stress in wheat. Indian J. Plant Physiol. 2017, 22, 365–368.
- Ristic, Z.; Momilović, I.; Bukovnik, U.; Prasad, P.V.V.; Fu, J.; Deridder, B.P.; Elthon, T.E.; Mladenov, N. Rubisco activase and wheat productivity under heat-stress conditions. J. Exp. Bot. 2009, 60, 4003–4014.
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681.
- Kumar, A.; Sahu, C.; Panda, P.A.; Biswal, M.; Sah, R.P.; Lal, M.K.; Baig, M.J.; Swain, P.; Behera, L.; Chattopadhyay, K.; et al. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J. Sci. Food Agric. 2020, 100, 1598–1607.
- Asthir, B. Protective mechanisms of heat tolerance in crop plants. J. Plant Interact. 2015, 10, 202–210.
- Pandey, R.; Lal, M.K.; Vengavasi, K. Differential response of hexaploid and tetraploid wheat to interactive effects of elevated and low phosphorus. Plant Cell Rep. 2018, 37, 1231–1244.
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological responses of forest trees to heat and drought. Plant Biol. 2006, 8, 556–571.
- Ihsan, M.Z.; Daur, I.; Alghabari, F.; Alzamanan, S.; Rizwan, S.; Ahmad, M.; Waqas, M.; Shafqat, W. Heat stress and plant development: Role of sulphur metabolites and management strategies. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 332–342.
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6.
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803.
- Narayanan, S.; Prasad, P.V.V.; Fritz, A.K.; Boyle, D.L.; Gill, B.S. Impact of high night-time and high daytime temperature stress on winter wheat. J. Agron. Crop Sci. 2015, 201, 206–218.
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19.
- Kumar, A.; Lal, M.K.; Kar, S.S.; Nayak, L.; Ngangkham, U.; Samantaray, S.; Sharma, S.G. Bioavailability of iron and zinc as affected by phytic acid content in rice grain. J. Food Biochem. 2017, 41, e12413.
- Todaka, D.; Nakashima, K.; Maruyama, K.; Kidokoro, S.; Osakabe, Y.; Ito, Y.; Matsukura, S.; Fujita, Y.; Yoshiwara, K.; Ohme-Takagi, M.; et al. Rice phytochrome-interacting factor-like protein OsPIL1 functions as a key regulator of internode elongation and induces a morphological response to drought stress. Proc. Natl. Acad. Sci. USA 2012, 109, 15947–15952.
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Mangal, V.; Altaf, M.A.; Sharma, S.; Singh, B.; Kumar, M. Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol. Biol. 2021, 1–15.
- Xue, G.P.; Drenth, J.; McIntyre, C.L. TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L.) including previously unknown Hsf targets. J. Exp. Bot. 2015, 66, 1025–1039.
- Pradhan, G.P.; Prasad, P.V.V. Evaluation of wheat chromosome translocation lines for high temperature stress tolerance at grain filling stage. PLoS ONE 2015, 10, e0116620.
- Prieto, P.; Ochagavía, H.; Savin, R.; Griffiths, S.; Slafer, G.A. Dynamics of floret initiation/death determining spike fertility in wheat as affected by Ppd genes under field conditions. J. Exp. Bot. 2018, 69, 2633–2645.
- Paul, S.; Gogoi, N.; Sarma, B.; Baroowa, B. Biochemical changes in potato under elevated temperature. Indian J. Plant Physiol. 2014, 19, 36–42.
- Singh, A.; Khurana, P. Molecular and Functional Characterization of a Wheat B2 Protein Imparting Adverse Temperature Tolerance and Influencing Plant Growth. Front. Plant Sci. 2016, 7, 642.
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019, 25, 155–173.
- Collar, C.; Armero, E. Impact of heat moisture treatment and hydration level on physico-chemical and viscoelastic properties of doughs from wheat-barley composite flours. Eur. Food Res. Technol. 2018, 244, 355–366.
- Wang, X.; Hou, L.; Lu, Y.; Wu, B.; Gong, X.; Liu, M.; Wang, J.; Sun, Q.; Vierling, E.; Xu, S. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545.
- Wang, X.; Xu, Y.; Hu, Z.; Xu, C. Genomic selection methods for crop improvement: Current status and prospects. Crop J. 2018, 6, 330–340.
- Bastos, L.M.; Carciochi, W.; Lollato, R.P.; Jaenisch, B.R.; Rezende, C.R.; Schwalbert, R.; Vara Prasad, P.V.; Zhang, G.; Fritz, A.K.; Foster, C.; et al. Winter Wheat Yield Response to Plant Density as a Function of Yield Environment and Tillering Potential: A Review and Field Studies. Front. Plant Sci. 2020, 11, 54.
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic stress in crop species: Improving tolerance by applying plant metabolites. Plants 2021, 10, 186.
- Liu, C.; Sukumaran, S.; Claverie, E.; Sansaloni, C.; Dreisigacker, S.; Reynolds, M. Genetic dissection of heat and drought stress QTLs in phenology-controlled synthetic-derived recombinant inbred lines in spring wheat. Mol. Breed. 2019, 39, 34.
- Liu, H.; Able, A.J.; Able, J.A. Transgenerational effects of water-deficit and heat stress on germination and seedling vigour—New insights from durum wheat microRNAs. Plants 2020, 9, 189.
- Wijewardana, C.; Raja Reddy, K.; Jason Krutz, L.; Gao, W.; Bellaloui, N. Drought stress has transgenerational effects on soybean seed germination and seedling vigor. PLoS ONE 2019, 14, e0214977.
- Ibrahim, A.M.H.; Quick, J.S. Genetic control of high temperature tolerance in wheat as measured by membrane thermal stability. Crop Sci. 2001, 41, 1405–1407.
- Nayak, L.; Panda, D.; Dash, G.K.; Lal, M.K.; Swain, P.; Baig, M.J.; Kumar, A. A chloroplast Glycolate catabolic pathway bypassing the endogenous photorespiratory cycle enhances photosynthesis, biomass and yield in rice (Oryza sativa L.). Plant Sci. 2022, 314, 111103.
- Schreiber, U.; Berry, J.A. Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus. Planta 1977, 136, 233–238.
- Pradhan, G.P.; Prasad, P.V.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. High temperature tolerance in Aegilops species and its potential transfer to wheat. Crop Sci. 2012, 52, 292–304.
- Narayanan, S.; Prasad, P.V.V.; Welti, R. Alterations in wheat pollen lipidome during high day and night temperature stress. Plant Cell Environ. 2018, 41, 1749–1761.
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147.
- Cheabu, S.; Moung-Ngam, P.; Arikit, S.; Vanavichit, A.; Malumpong, C. Effects of Heat Stress at Vegetative and Reproductive Stages on Spikelet Fertility. Rice Sci. 2018, 25, 218–226.
- Saini, H.S.; Sedgley, M.; Aspinall, D. Effect of heat stress during floral development on pollen tube growth and ovary anatomy in wheat (Triticum aestivum L.). Aust. J. Plant Physiol. 1983, 10, 137–144.
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; American Society of Agronomy: Madison, WI, USA, 2015; Volume 1 advancesin, pp. 301–355. ISBN 9780891181880.
- García, G.A.; Dreccer, M.F.; Miralles, D.J.; Serrago, R.A. High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Glob. Chang. Biol. 2015, 21, 4153–4164.
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065.
- Mamrutha, H.M.; Rinki, K.; Venkatesh, K.; Gopalareddy, K.; Khan, H.; Mishra, C.N.; Kumar, S.; Kumar, Y.; Singh, G.; Singh, G.P. Impact of high night temperature stress on different growth stages of wheat. Plant Physiol. Rep. 2020, 25, 707–715.
More
