Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Camila Xu.
The active ultraviolet filters (UVFs) in sunscreens can be organic or inorganic and can reflect and scatter UVR, which protects human skin from direct sunlight radiation. However, sunscreen-derived inorganic UVFs are considered to be emerging contaminants; in particular, nZnO and nTiO2 UVFs have been shown to undergo absorption and bioaccumulation, release metal ions, and generate reactive oxygen species, which cause negative effects on aquatic organisms.
- cosmetics
- nanoparticles
- environmental behavior
- water
1. Introduction
Sunscreen is one of the personal care products (PCPs) used to provide protection against ultraviolet radiation (UVR, 10–400 nm) damage [1][2][3]. Recently, with rising production and consumption, sunscreens have been increasingly released into aquatic environments, including oceans, rivers, lakes, and other water bodies, via several means of discharge (e.g., wastewater treatment plant effluents, runoff input, and recreational activities) [4][5][6]. The rapid growth in global tourism, especially coastal and marine tourism, where the number of international tourists worldwide grew from 463 million in 1992 to 763 million in 2004 and is estimated to have reached 1.56 billion in 2020 [7], has contributed to the increasing application of sunscreen [7][8]. Moreover, in these tropical countries, at least 25% of the sunscreens applied to skin are eventually released into the ocean during water recreational activities [9], which could pose potential risks to the aquatic environment.
Sunscreen is a multicomponent product that contains both active ingredients to shield or reflect UVR and commodity coatings to prevent bleaching and the loss of color [10]. The active ultraviolet filters (UVFs) in sunscreens can be organic or inorganic and can reflect and scatter UVR, which protects human skin from direct sunlight radiation [11][12]. Typically, organic UVFs are called chemical filters, as their mode of action (MoA) is related to the chemical changes in their molecules that prevent UVR from reaching the skin. The European Union regulates and authorizes 26 types of organic UVFs [13], which are widely used and globally recognized. In 2018, the Environmental Working Group (EWG) reported that two-thirds of the 1300 sunscreen products available contain chemicals that the EWG has deemed to be harmful to the environment, which are predominantly organic UVFs [14]. Inorganic UVFs are called physical filters or mineral filters, as their MoA is associated with physical phenomena, such as the scattering and reflection of UVR [15][16][17][18][19][20]. Titanium dioxide (TiO2) and zinc oxide (ZnO) are the most widely used inorganic UVFs and are usually present in nanoparticle (NP) form, also known as nanosized TiO2 (nTiO2) and nanosized ZnO (nZnO), due to their greater dispersion and UV scattering superficial area [14]. Both nTiO2 and nZnO are semiconductors with wide band gaps that can effectively shield UV light.
The adverse environmental effects of organic UVFs, including the bleaching effect on coral reefs and the negative hormonal effects on marine animals, were reviewed in a recent study [21]. The ecological risks of organic UVFs have resulted in warnings and restrictions on the application of chemical substances. The Hawaiian state legislature passed a bill on 1 May 2018 that bans the sale and distribution of sunscreens that contain certain organic UVFs (oxybenzone and octinoxate), which is anticipated to become effective in 2021 [22]. In addition, the EWG began to push the Food and Drug Administration in 2007 to update and improve cosmetic product regulations by urging the agency to set stricter standards to better protect public health [14].
Due to the ecotoxicological risks of organic UVFs, using inorganic UVFs for replacement has become a topic of interest for both producers and consumers. Although organic UVFs have dominated the market for PCPs in the past, inorganic UVFs as substitutions are increasing due to their broad UV spectrum protection and limited skin penetration and health risks [23][24]. It is believed that 60% of nTiO2 and 80% of nZnO produced globally are used in cosmetic products [25][26]. With the increasing production and application, the discharge of inorganic UVFs into environments is inevitable. In the United States, hundreds of tons of TiO2 and ZnO are disposed of in the environment every year [27]. To date, studies have shown that inorganic UVFs have been detected in marine waters, sediments, and organisms at increasing concentrations [1]. For example, Botta et al. [28] estimated that in reef areas, 36–56 tons of TiO2 were released from sunscreens, where the concentration of TiO2 could reach tens of milligram liters in surface microlayer [4]. Inorganic UVFs are prone to persisting in the environment due to continuous emissions and refractory degradation, which pose health threats to aquatic organisms at different trophic levels.
2. Inorganic Ultraviolet Filters (UVFs) in Aquatic Environments
2.1. Sources and Occurrences
UVFs have been detected in surface waters [29], urban groundwater [30], sediments [31][32][33], marine water, and biota [1][34]. The environmental sources and distribution of organic UVFs have been well reviewed in recent years [1][34]. However, very little is known about the occurrences and distributions of the two increasingly used inorganic UVFs (nTiO2 and nZnO). It has been shown that these substances are released into waters, either directly through human activities or indirectly through wastewater treatment plant (WWTP) drainage and atmospheric deposition (shown in Figure 1) [11][29][35]. Some studies have indicated that there is a direct relationship between the amounts of sunscreen components in waters and recreational activities, such as swimming, diving, surfing, etc [4][36][37]. In addition, the effluents of WWTPs and domestic sewage indirectly release UVFs, as sunscreen components cannot be completely removed [6][11]. Atmospheric aerosols containing UVFs may occur from different sources, including directly after spraying sunscreen on the skin, with effluents from WWTPs, and indirectly with the incineration of WWTP sludge.
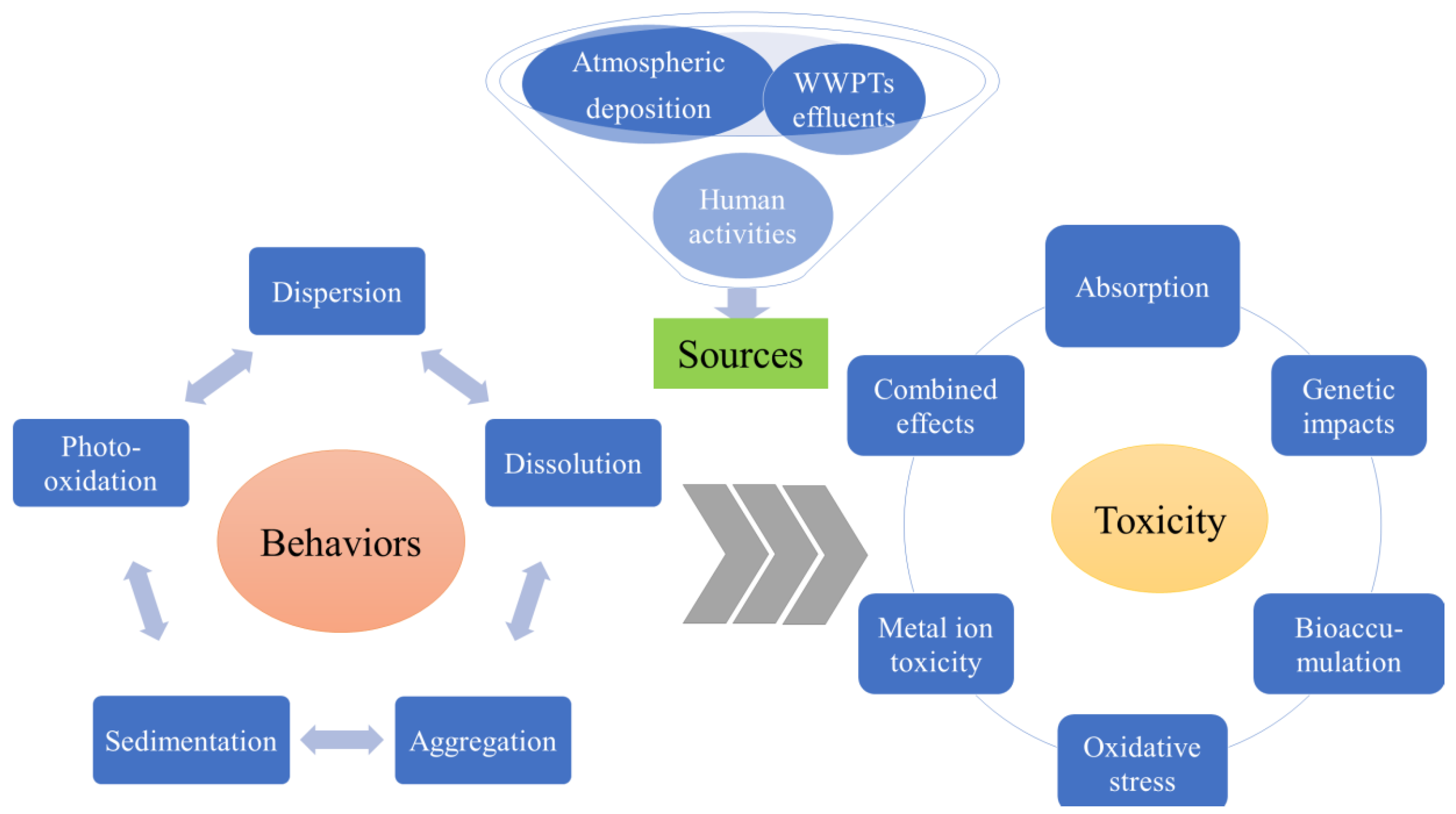 Figure 1. The sources, behaviors, and toxicity of sunscreen-derived inorganic UVFs in aquatic environments.
Figure 1. The sources, behaviors, and toxicity of sunscreen-derived inorganic UVFs in aquatic environments.
 Figure 1. The sources, behaviors, and toxicity of sunscreen-derived inorganic UVFs in aquatic environments.
Figure 1. The sources, behaviors, and toxicity of sunscreen-derived inorganic UVFs in aquatic environments.According to a survey study, there are approximately 16,000–25,000 tons per annual (t/a) of sunscreens that contain nTiO2 in tropical countries, and at least 25% of sunscreen applied to the skin enters the ocean during water recreational activities [9]. It is estimated that the content of nTiO2 in sunscreens is approximately 4%, and the amount of nTiO2 released annually is approximately 160–250 t in these tropical countries [1][38]. Specifically, Sánchez-Quiles and Tovar-Sánchez [9] estimated that over 4 kg of nTiO2 can be released from sunscreen into seawater during a summer day on a tropical touristic beach. Another study suggested that the recreational activities that take place at Old Danube Lake (Vienna, Austria) may involve the consumption of sunscreen of 8.1 t per year, and they estimated that 94.5 kg of TiO2 per year may be released into lake waters [39]. A recent study has shown that inorganic UVFs present in the formulation of sunscreens are detected in nearshore water and are concentrated in the surface microlayer that ranges from 6.9 to 37.6 mg/L for TiO2 and from 1.0 to 3.3 mg/L for ZnO [4].
2.2. Environmental Behaviors
The specific behavior of inorganic UVFs released from sunscreens into aquatic environments has not been well addressed. As sunscreen is a complex chemical mixture; once it is in water, the inorganic UVFs released from sunscreen are complex and can exist in the form of aggregates of various complex components [40][41], including surface-modified complexes or raw NPs. For raw NPs, their environmental fate generally includes dispersing, aggregating, and dissolving/releasing metal ions and settling onto sediments or being absorbed and bioaccumulated by organisms (shown in Figure 1) [28][39][42]. Many studies have confirmed that nZnO UVFs rapidly dissolve in water and form hydrated Zn2+ cations [43][44]. Other inorganic UVFs, e.g., nTiO2, are regarded as relatively stable and rather insoluble in water [45]. Thus, these UVFs tend to aggregate into larger particles, which remain suspended or precipitate to the bottom of the aquatic environment. In general, the higher the content of UVFs, the higher the SPR the sunscreen obtained. For organic UVFs, they absorb UVR, thus their spectral characteristics determined the absorbance of UVR as well as the sun protection factor (SPR); most of them are photo-instability effects related to UVR exposure [46]. For inorganic UVFs, they mean to scatter and reflect UVR; thus, they are more stable than organic UVFs, but their particle size would affect the SPR and transparency (aesthetics of the products), thus most inorganic UVFs are nanosized. The stability of physical sunscreens was influenced by the coating materials, with these organic materials in physical sunscreens tend to perform photodegradation and photo-instability effects related to UVR exposure, thus making inorganic UVFs easier to bear in the environment [46]. In addition, photooxidation and photodegradation are also proposed to occur when inorganic UVFs are exposed to sunlight. Inorganic UVFs, including nTiO2 and nZnO, are often used as photocatalytic materials; once released into water, they can be photooxidized during irradiation by ultraviolet light and generate hole-electron pairs; reactive oxygen species (ROS) are produced when hole-electron pairs react with H2O or O2 on the surface of NPs, which also decreases the particle size and produces more ROS [47][48]. Studies have shown that inorganic UVFs are photooxidized, produce ROS, and cause photocatalytic toxicity to aquatic organisms [49]. In addition to these behaviors, inorganic UVFs easily settle into sediments due to gravitational force, thereby aggregating into larger NPs. UVFs, both the organic and inorganic varieties, are absorbed or captured by aquatic organisms during the above processes, which causes damage to organisms and even bioaccumulation in organisms or sediments in the water. It is recently found that physical sunscreens and related inorganic UVFs exhibit bioattachment on the surfaces of button coral and cause significant growth inhibition and expulsion of zooxanthellae (Symbiodinium sp., unpublished data), which demonstrates the importance of further exploring the environmental fate of inorganic UVF-containing cosmetic products and the derived UVFs.
The nTiO2 and nZnO were dispersed (partial dissolved) in physical sunscreens during the manufacturing process, which would be modified first sometimes. Thus inorganic UVFs in sunscreens often exist as surface-modified complexes. For surface-modified complexes, their potential environmental behavior presents some differences that need to be discussed. Primarily, coexisting surface coatings affect the fate of NPs to some extent. In addition to UVFs, sunscreens also contain other ingredients, such as preservatives (e.g., paraben derivates) [50], coloring agents (e.g., ammonium sulfate, ferric ammonium ferrocyanide, copper powder, and iron) [51], film-forming agents (e.g., acrylates and acrylamides) [52], surfactants, chelators, viscosity controllers (e.g., potassium cetyl phosphate and pentasodium ethylenediamine tetramethylene phosphonate), and fragrances [53]. Some of these ingredients have been detected in coastal waters [54][55][56]. Thus, nTiO2 (and nZnO) may be present in the form of bare or coated NPs in the aquatic environment, and their dimension, shape, crystal phase, and surface area vary among different sunscreen products [27]. A recent study showed that sunscreen-derived nTiO2 exhibits a larger particle size but a smaller hydrodynamic diameter and lower zeta potential than industrial uncoated nTiO2, which exhibits significant aggregation [57]. In contrast, the presence of carboxymethyl cellulose (CMC) or polyvinylpyrrolidone (PVP) significantly enhances the stability of uncoated nTiO2, as determined by the zeta potential values measured at pH 7, with substantial shape changes that result in spherical particles and relatively small nTiO2 sizes [57]. Similar substantial shape transformations induced by stabilizers have been found in other studies [58][59]. Inorganic UVFs generally have a small particle size, strong hydrophobicity, and are insoluble in water; thus, Brownian motion, eddy motion, and runoff shear force result in some inorganic UVF particles remaining in suspension [60]. Engineered polymers or organic and inorganic substances that serve as coating materials or act as stabilizers have been found to modify the physicochemical properties of raw NPs, thereby affecting particle stability and mobility through electrostatic repulsion [61][62][63] and by maintaining the dispersion of nanosized inorganic UVFs. For example, nTiO2 has been found to be fully dispersed and stabilized in natural water that contains organic materials [64]. Therefore, the stability of inorganic UVFs depends on their physicochemical properties and coating materials [27][57].
An early study indicated that eight of nine commercial sunscreen products are coated with nonvolatile inorganic residues, typically Al2O3 or SiO2, to minimize the photochemical activity of TiO2 [27]. Adsorbed or covalently bonded surfactants affect aggregation stability by increasing the surface charge and electrostatic repulsion or by reducing the interfacial energy between the particles and the solvent [65]. The interaction between steric repulsion and universal Coulomb attraction is caused by the surface coating layers, which may profoundly affect the aggregation kinetics. However, a recent study showed that sodium citrate provides higher stability for spherical nTiO2 than PVP, sodium dodecyl sulfate, and polyethylene glycol, since sodium citrate results in lower critical coagulation concentrations [66]. Additionally, another study showed that the addition of coating materials such as CMC, PVP, and silica prevents significant TiO2 aggregation by facilitating dispersion [60]. These stabilizers change the physicochemical properties (particle sizes and zeta potential) of nTiO2 and produce a stable TiO2 suspension with a cluster size smaller than that of uncoated nTiO2 because they play the role of a dispersant that prevents nanoparticle aggregation [57]. A decrease in particle size results in a higher proportion of atoms on the particle surface, which alters the electronic structure, surface charge, and final degree of aggregation [67]. Small particles with high surface energy aggregate more readily than larger particles since aggregation reduces the free energy in the NP system.
It has been revealed that the dissolution of inorganic UVFs depends on the solubility of the materials themselves and on the concentration gradient in water [68][69]. For example, nZnO releases more Zn ions in seawater with a higher ionic strength than in fresh water [70]. Moreover, the dissolution of inorganic UVFs is clearly affected by the physicochemical properties of the material, such as the particle size, shape, and surface coating. Generally, the solubility of NPs is higher than that of the bulk phase because the decreased size increases the specific surface areas and the enthalpies of the formation of the ions [71]. Fairbairn et al. [72] also pointed out that nZnO is more easily dissolved in sea water than ZnO with ordinary particle sizes or Fe-doped nZnO. However, for nZnO, the impact of different sizes on dissolution is not as obvious for nanosized, bulk, or large particles due to the high solubility of ZnO, which can exhibit up to 80% dissolution [69][73][74]. Additionally, the shapes of NPs have been shown to affect both the rates of dissolution and the equilibrium concentrations [14]. The dissolution rate for spherical nCuO is faster than that of rod and spindle nCuO [75], while spherical nZnO induces lower toxicity than rod-shaped nZnO because the actual Zn ion concentration that results from the dissolution of rod-shaped nZnO is much higher than that of spherical nZnO [76].
Quite often, the dissolution rate of inorganic UVFs significantly decreases in the presence of surface coatings because the surface coating acts as a physical barrier or shield that prevents electrons or photons from reaching the NP surface [77]. In sunscreens, photoactivity problems may arise if particles are not treated with coatings, and manufacturers commonly employ inert surface coatings that dramatically reduce the potential for photoactivity; existing data suggests that these surface coatings reduce UV reactivity by as much as 99% [40][41]. However, organic coatings slow the dissolution process relative to that of uncoated ZnO but lead to an increased concentration of Zn2+ at equilibrium [78]. Otherwise, if the coatings are not stable or if manufacturers use forms of ZnO or TiO2 that are not optimized for stability and sun protection, then sunscreens may not be protective [14]. These results suggest that inorganic UVFs might input substantial amounts of free metals into an aquatic environment and pose a toxicity risk to aquatic ecosystems.
In addition to the influence of internal NP properties, external environmental factors such as light, pH, and natural organic matter (NOM) can also make a difference. The interaction energy barrier decreases with a decreasing particle size according to the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, and it is affected by the properties of the primary NPs (e.g., size, shape, chemical composition, and surface coatings), solution chemistries (e.g., pH, ionic identity, electrolyte patterns, and reactions with NOM), and environmental conditions (e.g., temperature and dissolved oxygen level) [69][79]. For example, a large proportion of nZnO dissolves at a limit close to the solubility limit of ZnO(s) at a high pH of approximately 8.2, and both visible and UV light facilitate nZnO dissolution at lower pH values that range from 4.8 to 6.5 [80]. Light warms the water, enhances the release rates of inorganic UVFs, shortens the equilibrium time and even increases equilibrium concentrations [62]. Moreover, inorganic UVFs generate ROS under irradiation with visible and UV light; this results in the oxidation of metal ions and surface organic compounds, which increases the dissolution rates due to the decomposition of surface coatings and loss of the stabilizing effect of dissolved organic matter. The influence of solution properties on the dissolution of inorganic UVFs is dynamic and complex [62].
2.3. Substantial Environmental Impacts
The discharge of inorganic UVFs from sunscreens into waters is concomitant with the input of several other constituents, including nutrients (e.g., silicates, phosphates, and nitrates), metals (e.g., Al, Cd, Cu, Co, Mn, Mo, Ni, Pb, and Ti), and coating materials (e.g., preservatives, coloring agents, film-forming agents, surfactants, and stabilizers). Many of these coexisting substances are persistent; therefore, their effects might last beyond the most recent period of sunscreen use. These additional constituents influence the bioavailability and degradability of sunscreen ingredients since the biogeochemical routes into environmental media (water, sediment, and biota) and the hydrophobicity or hydrophilicity of the substances contained in sunscreens are diverse and complex [1][81]. Moreover, the effects of sunscreen contamination (especially from commercial formulations instead of individual compounds or ingredients) are sometimes difficult to perceive in laboratory studies because of their complex matrix [82][83] and unknown composition [84]. Additionally, because of the diverse formats of sunscreens (e.g., cream, gel, spray, and oil), their dilution and release of UVFs into water are different, as are their bioavailabilities and toxicities [4][85].
It is likely that environmental exposure to inorganic UVFs and the chemicals contained therein results from the production and consumption of sunscreens. Studies have indicated that UVFs and other ingredients from sunscreens have been detected in the tissues of marine organisms, such as clams, oysters, gastropods, and fish [86][87], and have shown toxicity in some aquatic species, such as the crustacean Daphnia pulex and the fish Danio rerio [88][89]. Rodríguez-Romero et al. [90] demonstrated with laboratory experiments and field measurements that sunscreens are an important source of nutrients, such as nitrogen compounds (NO3−, NO2−, and NH4+) and phosphate (PO43−) in coastal marine environments, raising the possibility of algal blooms in oligotrophic waters. More specifically, some concentrations of the compounds (e.g., those of PO43-, NH4+, NO3−, and Ti) released into water vary during the course of a day, which is known to be associated with variations in beach-goer activities and changes in solar radiation [4]. Sunscreens have also been identified as sources of high-risk metal substances [91], many of which (e.g., Al, Zn, Mg, Fe, Mn, Cu, Cr, and Pb) have been detected and quantified in aquatic environments [4][92]. Moreover, the organic components of sunscreens are readily removed from particle surfaces [93][94], which leaves the inorganic UVFs exposed to the surrounding environment. Although the ecological relevance of this input has not been well reviewed, Tovar-Sánchez et al. [4] suggested that it could enhance primary production in the oligotrophic waters of the Mediterranean Sea.
In addition to the direct output of soluble substances from sunscreens, some indirect metabolites are also produced in the water environment under sunlight. A study carried out on a touristic beach indicated that both temporal (daily) and vertical (water column) distributions of H2O2 concentrations generated by inorganic UVFs (nTiO2 and nZnO) were present in marine waters [9]. According to the authors, the concentrations of H2O2 found within the top centimeter of the surface layer were up to 41.6% higher than those in the immediate subsurface waters [9]. Similarly, a large number of studies have indicated that nTiO2 and nZnO produce ROS under sunlight exposure and induce oxidative stress in organisms [62][95][96][97][98]. Therefore, more reliable information is required on the role of sunlight in the release of the main ingredients and byproducts of sunscreens into water.
Accordingly, sunscreen-derived inorganic UVFs are very likely to be released into the main water bodies of lakes, rivers, and oceans but do not remain suspended for a long time, with the most likely fates being aggregation, dissolution, and settling onto the sediments due to the water chemistry conditions and the presence of natural colloids. However, their environmental behaviors will be affected by the surface coating and various physical and chemical factors, such as ocean currents, waves, and high salinity, and they will undergo complex aggregation and dissolution reactions; moreover, their structural form, distribution, and toxic effects will constantly change. Nevertheless, these behaviors and transformation processes for inorganic UVFs must influence their bioavailability and toxicity, which cause great impacts on natural aquatic ecosystems [80].
References
- Sanchez-Quiles, D.; Tovar-Sanchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170.
- Lim, H.W.; Draelos, Z.D. Clinical Guide to Sunscreens and Photoprotection; Informa Health Care: New York, NY, USA, 2008.
- Urbach, F. The historical aspects of sunscreens. J. Photochem. Photobiol. B Biol. 2001, 64, 99–104.
- Tovar-Sanchez, A.; Sanchez-Quiles, D.; Basterretxea, G.; Benede, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451.
- Gormsen, E. The impact of tourism on coastal areas. GeoJournal 1997, 42, 39–54.
- Li, W.; Ma, Y.; Guo, C.; Hu, W.; Liu, K.; Wang, Y.; Zhu, T. Occurrence and behavior of four of the most used sunscreen UV filters in a wastewater reclamation plant. Water Res. 2007, 41, 3506–3512.
- Honey, M.; Krantz, D. Global Trends in Coastal Tourism; Center on Ecotourism and Sustainable Development: Washington, DC, USA, 2007.
- Hall, C.M. Trends in ocean and coastal tourism: The end of the last frontier? Ocean. Coast. Manag. 2001, 44, 601–618.
- Sanchez-Quiles, D.; Tovar-Sanchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042.
- Manová, E.; von Goetz, N.; Hauri, U.; Bogdal, C.; Hungerbühler, K. Organic UV filters in personal care products in Switzerland: A survey of occurrence and concentrations. Int. J. Hyg. Environ. Health 2013, 216, 508–514.
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TrAC Trends Anal. Chem. 2007, 26, 360–374.
- Salvador, A.; Chisvert, A. Analysis of Cosmetic Products; Elsevier Science: Amsterdam, The Netherlands; London, UK, 2011.
- Zhu, X.S.; Huang, J.Y.; Lü, X.H.; Du, Y.F.; Cai, Z.H. Fate and Toxicity of UV Filters in Marine Environments. Huanjing Kexue/Environ. Sci. 2018, 39, 2991–3002.
- Environmental Working Group. EWG’s Sunscreen Guide. 2019. Available online: https://www.ewg.org/sunscreen/report/executive-summary/ (accessed on 20 December 2020).
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Sunscreens–what’s important to know. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1110–1119.
- Jin, C.-Y.; Zhu, B.S.; Wang, X.-F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877.
- Seite, S.; Colige, A.; Piquemal-Vivenot, P.; Montastier, C.; Fourtanier, A.; Lapiere, C.; Nusgens, B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol. Photoimmunol. Photomed. 2000, 16, 147–155.
- van der Pols, J.C.; Williams, G.M.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol. Prev. Biomark. 2006, 15, 2546–2548.
- Balogh, T.S.; Velasco, M.; Pedriali, C.A.; Kaneko, T.M.; Baby, A.R. Ultraviolet radiation protection: Current available resources in photoprotection. BrasDermatol 2011, 86, 732–742.
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A review of the current and future technologies. Dermatol. Ther. 2010, 23, 31–47.
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244.
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446.
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594.
- Watkinson, A.C.; Bunge, A.L.; Hadgraft, J.; Lane, M.E. Nanoparticles do not penetrate human skin-a theoretical perspective. Pharm. Res. 2013, 30, 1943–1946.
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1109.
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447.
- Lewicka, Z.A.; Benedetto, A.F.; Benoit, D.N.; William, W.Y.; Fortner, J.D.; Colvin, V.L. The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens. J. Nanoparticle Res. 2011, 13, 3607.
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.-Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550.
- Tsui, M.M.; Leung, H.; Wai, T.-C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65.
- Jurado, A.; Gago-Ferrero, P.; Vàzquez-Suñé, E.; Carrera, J.; Pujades, E.; Díaz-Cruz, M.S.; Barceló, D. Urban groundwater contamination by residues of UV filters. J. Hazard. Mater. 2014, 271, 141–149.
- Amine, H.; Gomez, E.; Halwani, J.; Casellas, C.; Fenet, H. UV filters, ethylhexyl methoxycinnamate, octocrylene and ethylhexyl dimethyl PABA from untreated wastewater in sediment from eastern Mediterranean river transition and coastal zones. Mar. Pollut. Bull. 2012, 64, 2435–2442.
- Barón, E.; Gago-Ferrero, P.; Gorga, M.; Rudolph, I.; Mendoza, G.; Zapata, A.M.; Díaz-Cruz, S.; Barra, R.; Ocampo-Duque, W.; Páez, M. Occurrence of hydrophobic organic pollutants (BFRs and UV-filters) in sediments from South America. Chemosphere 2013, 92, 309–316.
- Rodil, R.; Moeder, M. Development of a simultaneous pressurised-liquid extraction and clean-up procedure for the determination of UV filters in sediments. Anal. Chim. Acta 2008, 612, 152–159.
- Rainieri, S.; Barranco, A.; Primec, M.; Langerholc, T. Occurrence and toxicity of musks and UV filters in the marine environment. Food Chem. Toxicol. 2017, 104, 57–68.
- Díaz-Cruz, M.S.; Barceló, D. Chemical analysis and ecotoxicological effects of organic UV-absorbing compounds in aquatic ecosystems. TrAC Trends Anal. Chem. 2009, 28, 708–717.
- Balmer, M.E.; Buser, H.R.; Muller, M.D.; Poiger, T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ. Sci. Technol. 2005, 39, 953–962.
- Poiger, T.; Buser, H.R.; Balmer, M.E.; Bergqvist, P.A.; Muller, M.D. Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951–963.
- Wong, S.W.; Leung, P.T.; Djurisic, A.B.; Leung, K.M. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010, 396, 609–618.
- Gondikas, A.P.; Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old Danube recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422.
- SCCNFP, Opinion Concerning Titanium Dioxide. Opinion: European Commission—The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Sci. Comm. Cosmet. Prod. Non-Food Prod. Intend. Consum. 2000, 0005/98, 2–43. Available online: https://ec.europa.eu/health/ph_risk/committees/sccp/documents/out135_en.pdf (accessed on 15 November 2021).
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.; Pernodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 5, 511–520.
- von der Kammer, F.; Ottofuelling, S.; Hofmann, T. Assessment of the physico-chemical behavior of titanium dioxide nanoparticles in aquatic environments using multi-dimensional parameter testing. Environ. Pollut. 2010, 158, 3472–3481.
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637, 1279–1285.
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total Environ. 2014, 476, 657–666.
- Schmidt, J.; Vogelsberger, W. Dissolution kinetics of titanium dioxide nanoparticles: The observation of an unusual kinetic size effect. J. Phys. Chem. B 2006, 110, 3955–3963.
- Garoli, D.; Pelizzo, M.G.; Bernardini, B.; Nicolosi, P.; Alaibac, M. Sunscreen tests: Correspondence between in vitro data and values reported by the manufacturers. J. Dermatol. Sci. 2008, 52, 193–204.
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative determination of OH radical generation and its cytotoxicity induced by TiO2–UVA treatment. Toxicology 2002, 16, 629–635.
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90.
- Cunningham, B.; Torres-Duarte, C.; Cherr, G.; Adams, N. Effects of three zinc-containing sunscreens on development of purple sea urchin (Strongylocentrotus purpuratus) embryos. Aquat. Toxicol. 2020, 218, 105355.
- Peck, A.M. Analytical methods for the determination of persistent ingredients of personal care products in environmental matrices. Anal. Bioanal. Chem. 2006, 386, 907–939.
- Valet, B.; Mayor, M.; Fitoussi, F.; Capellier, R.; Dormoy, M.; Ginestar, J. Colouring agents in cosmetic products (excluding hair dyes): Types of decorative cosmetic products. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 141–152.
- Quartier, S.; Garmyn, M.; Becart, S.; Goossens, A. Allergic contact dermatitis to copolymers in cosmetics–case report and review of the literature. Contact Dermat. 2006, 55, 257–267.
- Tønning, K.; Jacobsen, E.; Pedersen, E.; Strange, M.; Poulsen, P.B.; Møller, L.; Boyd, H.B. Survey and Health Assessment of the exposure of 2 year-olds to chemical substances in Consumer Products; Danish Ministry of the Environment, Environmental Protection Agency: Odense, Denmark, 2009; Chapter 5; p. 91.
- Vecchiato, M.; Gregoris, E.; Barbaro, E.; Barbante, C.; Piazza, R.; Gambaro, A. Fragrances in the seawater of Terra Nova Bay, Antarctica. Sci. Total Environ. 2017, 593, 375–379.
- Kung, T.A.; Lee, S.H.; Yang, T.C.; Wang, W.H. Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan. Sci. Total Environ. 2018, 635, 1302–1307.
- Zhao, X.; Qiu, W.; Zheng, Y.; Xiong, J.; Gao, C.; Hu, S. Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China. Ecotoxicol. Environ. Saf. 2019, 180, 43–52.
- Baek, S.; Joo, S.H.; Blackwelder, P.; Toborek, M. Effects of coating materials on antibacterial properties of industrial and sunscreen-derived titanium-dioxide nanoparticles on Escherichia coli. Chemosphere 2018, 208, 196–206.
- Othman, S.H.; Abdul Rashid, S.; Mohd Ghazi, T.I.; Abdullah, N. Dispersion and stabilization of photocatalytic TiO2 nanoparticles in aqueous suspension for coatings applications. J. Nanomater. 2012, 2012, 718214.
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017.
- Peng, C.; Zhang, W.; Gao, H.; Li, Y.; Tong, X.; Li, K.; Zhu, X.; Wang, Y.; Chen, Y. Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments. Nanomaterials 2017, 7, 21.
- Jafry, H.R.; Liga, M.V.; Li, Q.; Barron, A.R. Simple route to enhanced photocatalytic activity of P25 titanium dioxide nanoparticles by silica addition. Environ. Sci. Technol. 2010, 45, 1563–1568.
- Dalai, S.; Pakrashi, S.; Kumar, R.S.; Chandrasekaran, N.; Mukherjee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 2012, 1, 116–130.
- Wang, J.; Fan, Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: Size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014, 15, 22258–22278.
- Planchon, M.; Ferrari, R.; Guyot, F.; Gélabert, A.; Menguy, N.; Chanéac, C.; Thill, A.; Benedetti, M.F.; Spalla, O. Interaction between Escherichia coli and TiO2 nanoparticles in natural and artificial waters. Colloids Surf. B Biointerfaces 2013, 102, 158–164.
- Rosen, M.; Kunjappu, J. Adsorption of surface-active agents at interfaces: The electrical double layer. Surfactants Interfacial Phenom. 2004, 4, 98.
- Raza, G.; Amjad, M.; Kaur, I.; Wen, D. Stability and aggregation kinetics of titania nanomaterials under environmentally realistic conditions. Environ. Sci. Technol. 2016, 50, 8462–8472.
- Waychunas, G.A.; Kim, C.S.; Banfield, J.F. Nanoparticulate iron oxide minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms. J. Nanoparticle Res. 2005, 7, 409–433.
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006, 90, 23–32.
- Bian, S.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068.
- Zhang, L.; Li, J.; Yang, K.; Liu, J.; Lin, D. Physicochemical transformation and algal toxicity of engineered nanoparticles in surface water samples. Environ. Pollut. 2016, 211, 132–140.
- Gilbert, B.; Huang, F.; Zhang, H.; Waychunas, G.A.; Banfield, J.F. Nanoparticles: Strained and stiff. Science 2004, 305, 651–654.
- Fairbairn, E.A.; Keller, A.A.; Mädler, L.; Zhou, D.; Pokhrel, S.; Cherr, G.N. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571.
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490.
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138.
- Misra, S.K.; Nuseibeh, S.; Dybowska, A.; Berhanu, D.; Tetley, T.D.; Valsami-Jones, E. Comparative study using spheres, rods and spindle-shaped nanoplatelets on dispersion stability, dissolution and toxicity of CuO nanomaterials. Nanotoxicology 2014, 8, 422–432.
- Lee, J.; Kang, B.; Hicks, B.; Chancellor Jr, T.F.; Chu, B.H.; Wang, H.-T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749.
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2012, 28, 1095–1104.
- Gelabert, A.; Sivry, Y.; Ferrari, R.; Akrout, A.; Cordier, L.; Nowak, S.; Menguy, N.; Benedetti, M.F. Uncoated and coated ZnO nanoparticle life cycle in synthetic seawater. Environ. Toxicol. Chem. 2014, 33, 341–349.
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment; ACS Publications: Washington, DC, USA, 2012.
- Odzak, N.; Kistler, D.; Sigg, L. Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments. Environ. Pollut. 2017, 226, 1–11.
- Fel, J.-P.; Lacherez, C.; Bensetra, A.; Mezzache, S.; Béraud, E.; Léonard, M.; Allemand, D.; Ferrier-Pagès, C. Photochemical response of the scleractinian coral Stylophora pistillata to some sunscreen ingredients. Coral Reefs 2019, 38, 109–122.
- Rodil, R.; Moeder, M.; Altenburger, R.; Schmitt-Jansen, M. Photostability and phytotoxicity of selected sunscreen agents and their degradation mixtures in water. Anal. Bioanal. Chem. 2009, 395, 1513–1524.
- Paredes, E.; Perez, S.; Rodil, R.; Quintana, J.B.; Beiras, R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere 2014, 104, 44–50.
- Sureda, A.; Capo, X.; Busquets-Cortes, C.; Tejada, S. Acute exposure to sunscreen containing titanium induces an adaptive response and oxidative stress in Mytillus galloprovincialis. Ecotoxicol. Environ. Saf. 2018, 149, 58–63.
- Araujo, C.V.M.; Rodriguez-Romero, A.; Fernandez, M.; Sparaventi, E.; Medina, M.M.; Tovar-Sanchez, A. Repellency and mortality effects of sunscreens on the shrimp Palaemon varians: Toxicity dependent on exposure method. Chemosphere 2020, 257, 127190.
- Kim, J.-W.; Isobe, T.; Ramaswamy, B.R.; Chang, K.-H.; Amano, A.; Miller, T.M.; Siringan, F.P.; Tanabe, S. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an ultra-fast liquid chromatography-tandem mass spectrometry. Chemosphere 2011, 85, 751–758.
- Nakata, H.; Murata, S.; Filatreau, J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environ. Sci. Technol. 2009, 43, 6920–6926.
- Fent, K.; Chew, G.; Li, J.; Gomez, E. Benzotriazole UV-stabilizers and benzotriazole: Antiandrogenic activity in vitro and activation of aryl hydrocarbon receptor pathway in zebrafish eleuthero-embryos. Sci. Total Environ. 2014, 482, 125–136.
- Kim, J.-W.; Chang, K.-H.; Isobe, T.; Tanabe, S. Acute toxicity of benzotriazole ultraviolet stabilizers on freshwater crustacean (Daphnia pulex). J. Toxicol. Sci. 2011, 36, 247–251.
- Rodríguez-Romero, A.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Tovar-Sánchez, A. Sunscreens as a New Source of Metals and Nutrients to Coastal Waters. Environ. Sci. Technol. 2019, 53, 10177–10187.
- Zmozinski, A.V.; Pretto, T.; Borges, A.R.; Duarte, A.T.; Vale, M.G.R. Determination of Pb and Cr in sunscreen samples by high-resolution continuum source graphite furnace atomic absorption spectrometry and direct analysis. Microchem. J. 2016, 128, 89–94.
- Zachariadis, G.; Sahanidou, E. Multi-element method for determination of trace elements in sunscreens by ICP-AES. J. Pharm. Biomed. Anal. 2009, 50, 342–348.
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.-Y. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010, 158, 3482–3489.
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chaneac, C.; Botta, C.; Chaurand, P.; Labille, J. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ. Sci. Technol. 2010, 44, 2689–2694.
- Chen, T.H.; Lin, C.C.; Meng, P.J. Zinc oxide nanoparticles alter hatching and larval locomotor activity in zebrafish (Danio rerio). J. Hazard. Mater. 2014, 277, 134–140.
- Zhu, X.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Technol. 2011, 45, 3753–3758.
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870.
- Zhu, X.; Zhou, J.; Cai, Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar. Pollut. Bull. 2011, 63, 334–338.
More
