Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Faryal Farooq Farooq.
Honey is a mixture of 25 sugars with other bioactive substances (i.e., organic acids, enzymes, antioxidants, and vitamins) and has been known as a highly nutritious functional food. Traditionally, it has been widely used in medicinal applications to cure various diseases. The effectiveness of honey in different applications has been used for its antimicrobial activity, absorption of hydrops, cleansing, removing odor, assisting granulation, recovery of nutrition, and formation of tissue and epithelium, which proved that honey has dehydrating and preserving properties to make it ideal for the cryopreservation of cells and tissues.
- honey
- cryopreservation
- extenders
- natural cryoprotectant
- fertility
1. Introduction
Humans have relied on nature through many ages as a source of several different traditional medicines and for healing diseases [1]. Honey is a sweet and viscous substance, produced by bees from flower nectar or honeydew. It is greatly appreciated, not only as food, but also as medicine [2]. The use of honey in preservation is expected, as its application has a long medicinal history. It is the most persistent and oldest natural sweetening agent, and its utilization has increased immensely in the last two decades, due to its high therapeutic properties and nutritional value [3,4][3][4]. Honey rich nutritious compounds (i.e., sugars, macro and microelements, and biologically active substances) are essential for the healthy human body’s needs [5,6][5][6].
Honey has been used effectively in different applications throughout human civilization, with strong evidence. It is supposed to have started with ancient Egyptians, before 4000 BC, and was used for 30 centuries to preserve their mummies in honey [7]. It has also been used in wound healing and to treat several diseases, such as cancer, cardiovascular, ulcer, diabetic, and gastrointestinal diseases [8]. Scientific discovery in modern medicines, from time to time after the 14th century, also laid an essential foundation in the preserving procedure of honey [9].
In the 19th and early 20th centuries, some modern preservation concepts were discovered by researchers who studied freezing, cold hardiness, and freezing tolerance in the environment. This discovery is now known as “cryopreservation” [10], which usually requires cryoprotectant (CPA) to survive the impact of low-temperature freezing [11], but successful cryopreservation of biological systems is limited, due to the cytotoxicity of CPAs in both vitrification and slow freezing [12,13][12][13]. The addition of sugar in the cryoprotective freezing medium is one of the approaches to overcome the problems that limit cell viability success after thawing [14].
Due to the cryoprotectant toxicity, there is always a need for non-toxic CPAs, as an alternative to store cells at liquid nitrogen temperature; that’s why researchers recently turned back to natural material honey. Honey is now relatively widely used by researchers because it is a natural component that does not require sterilization or cause considerable side effects, which makes it the most interesting natural remedy to preserve biological cells in cryopreservation. Natural honey contains 25 sugars, mainly (fructose and glucose) comprising of about 95% of its dry weight [8,15,16][8][15][16]. Previous studies have proved that the cell’s survival rate has improved more effectively by the addition of two sugars mixture (sucrose and glucose) in vitrification medium, rather than the addition of sucrose alone [14]. Besides a huge portion of saccharides, many other bioactive substances are also present in honey, such as vitamins, organic acids, antioxidants, and enzymes. Such an exceptional composition of honey provides several nutritional, biological, and pharmacological effects on living cells, such as anticancer, immunosuppressive, antioxidant and antitoxin, antimicrobial, anti-inflammatory, and antimutagenic activities [8,15,16,17,18,19,20][8][15][16][17][18][19][20].
There are limited, but encouraging, data concerning the use of honey as a natural cryoprotectant, at most in fertility preservation, which confirmed honey’s beneficial effect on the viability of cryopreserved sperm and embryos [21,22][21][22]. Honey has been supplemented to the freezing solution of semen in goats (Maidin et al. (2018)) [23], gourami (Abinawanto et al. (2017)) [24], Arabian stallions (Reda I. El-Sheshtawy et al. (2016)) [25], and African catfish (Z.A. Muchlisin et al. (2015)) [26], aiming to improve post-thaw semen characteristics. Furthermore, Bilal Alfoteisy et al. (2020) used honey in the vitrification solution of cow bovine oocytes to improve post-thaw oocyte viability and embryonic development [27], and Fatemeh Sarmadia et al. (2019) reported that the vitrified and warmed mouse embryos with honey-based vitrification solution improved hatching and re-expansion rate of blastocyst [22]. Despite these benefits, more research must be conducted to better understand honey as a natural cryoprotectant in cryopreservation. The aim of this review was to clarify the beneficial effect of honey as a natural cryoprotectant in cryopreservation and provide a direction for future researches, in order to improve the post-thaw quality and viability of other types of cells and tissue. There is no systematic and comprehensive review focused on honey as a natural cryoprotectant. Traditional and modern applications of honey in medicine, tissue regeneration, and cryopreservation are summarized at the end of Figure 1. In this review, (1) honey and its technique to detect adulteration, (2) honey in medicinal, tissue engineering, and cryobiology application, (3) the effect of natural cryoprotectant honey on the viability of sperm, embryos, and oocytes, and, at last, (4) the current challenges and future perspectives, related to honey, are briefly discussed, in order to motivate the flourishing development of cryopreservation in the field of cryobiology.
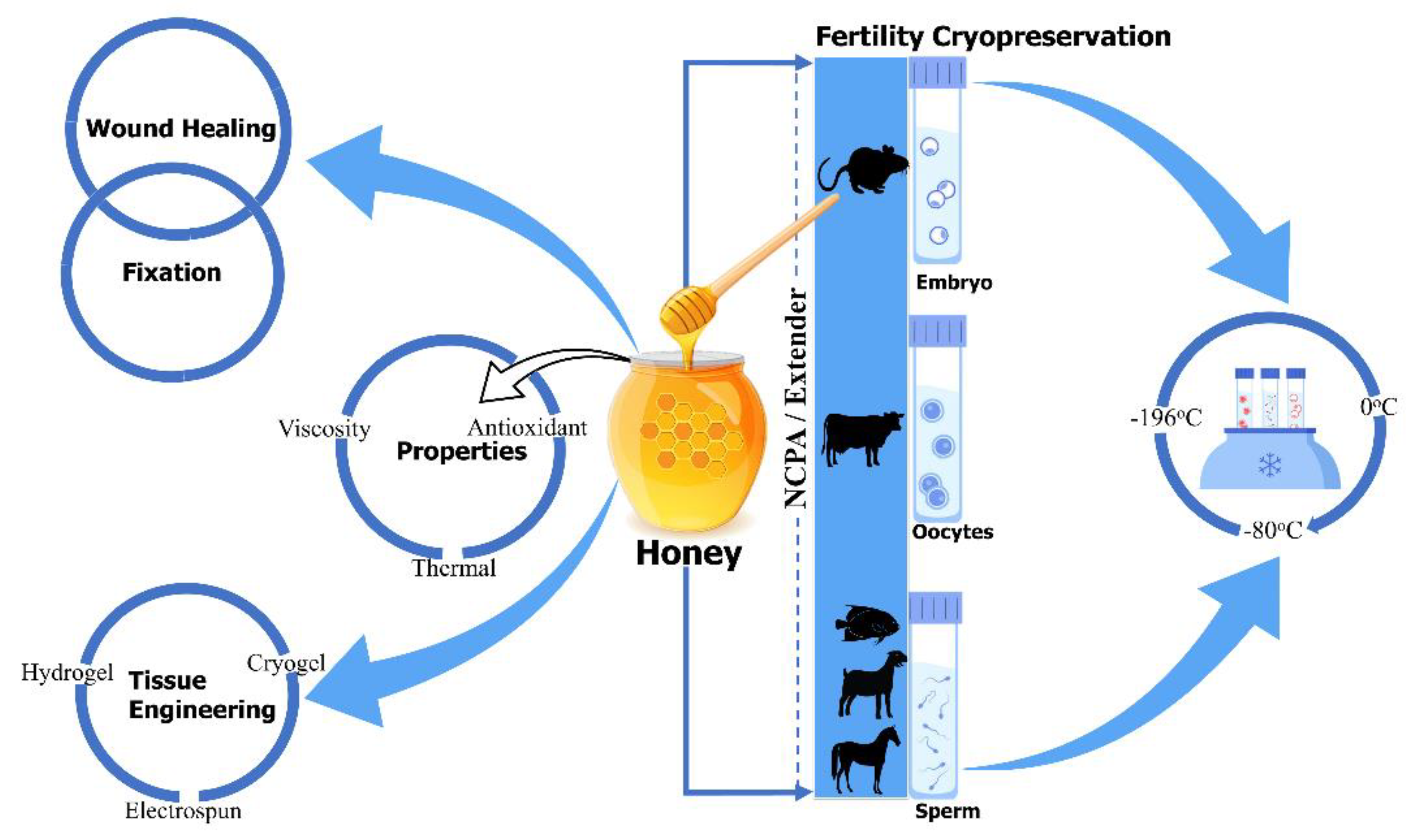
Figure 1.
Honey’s traditional and modern application in medicine, tissue regeneration, and cryopreservation.
2. Cryopreservation Related Properties
Many scientific articles have been reported, in regard to the various properties of honey. Considering the researchers’ increasing interest in using natural honey in the field of cryopreservation, wthe researchers have described, in detail, some highly efficient properties that benefit from low temperature freezing, such as rheological, thermal, and antioxidants of honey.
2.1. Rheological Property
Rheology is the study of the flow and deformation of a material under a given pressure [2]. Viscosity is the main rheological property of honey. It is a sticky and highly viscous liquid food because of its high sugar and low water contents. In the last decade, many studies have confirmed that the viscosity of different kinds of honey is greatly influenced by temperature and water content [52,53][28][29]. The viscosity of honey usually increases with decreasing temperature and water content because of high molecular friction and greater hydrodynamic forces [54,55,56,57][30][31][32][33]. Different equations, employed in several studies, can describe the viscosity–temperature connection. While the Arrhenius model was applied widely to describe the dependence of viscosity to temperature for many types of honey, some researchers proved that this model was not appropriate for all kinds of honey [52,54,58][28][30][34]. More clearly, some types of honey need other models (such as William–Landel–Ferry (WLF)) to show a logical relationship between their viscosities and temperatures. To describe the dynamic viscosity of honey, the WLF model uses glass-transition temperature (Tg) and viscosity in the glass state (hg) [52,56,59,60,61][28][32][35][36][37].
In many publications, honey is presented as a Newtonian fluid, from the rheological viewpoint, and characterized by constant viscosity (h) at a fixed temperature, which shows a linear relationship between shear stress (s) and shear rate (g) [54,62,63,64][30][38][39][40]. However, some kinds of honey were classified as non-Newtonian fluids. Some others reported a non-Newtonian behavior for certain honey types, including pseudoplastic for Galician (Spanish honey), thixotropy for a group of karvi, heather, manuka, buckwheat, and dilatancy for eucalyptus and Nigerian honey [65,66][41][42]. For non-Newtonian fluids, the shear rate (SR) ratio defines the fluid’s apparent viscosity (happ) at a constant temperature, similar to that of Newtonian fluids; however, this coefficient changes with shear rate, while the dynamic viscosity of Newtonian fluids is shear rate independent. Additionally, a thixotropic effect has also been observed by decrease in viscosity with time, at a constant shear rate and temperature [67,68,69][43][44][45]. The non-Newtonian behavior may be due to high molecular compounds, such as proteins or polysaccharides (dextrans), in their compositions, which also accounts for the usually observed thixotropic property [54,70][30][46]. Additionally reported, when some paste products’ molecular weights increased, due to different physical or biochemical processes, their viscosities and elasticities changed considerably. The increasingly high viscosity of honey, during lowering of the temperature, provides a protective barrier to prevent contamination and inhibit or retard ice crystal growth on a kinetic basis. Currently, this high viscous, non-toxic solution is significantly required in the field of cryopreservation, which may allow slow permeation and perfusion into cells/tissue, to provide protective benefits.
2.2. Thermal Property
The thermal properties of chemicals, food, and beverages must be known to perform the various heat transfer calculations involved in designing storage and refrigeration equipment and estimating procedure time for refrigerating, freezing, warming, or drying. It strongly depends on chemical composition and temperature. The thermo-physical properties, often required for heat transfer calculations, include density, specific heat, enthalpy, thermal conductivity, and thermal diffusivity [71][47]. DSC analysis showed the thermal nature of the samples. Glass transition temperature (Tg) is defined when an amorphous or partially amorphous substance undergoes the transition from a glassy solid to a rubbery viscous state at a specific temperature range, defined as a single temperature. This Tg is of great significance in determining honey’s efficiency, production, thermal protection, shelf life, and stability predictability [53][29].
In general, honey is an over-saturated sugar solution. The two main sugars in honey are fructose and glucose, and it varies with a different type of honey. Generally, the fructose and glucose ranges are 38% and 31% [31][48]. The balance of these two main sugars is the significant reason for the crystallization of honey, and each relative percentage determines whether it crystallizes rapidly or slowly. The water content in honey is lower when the percentage of glucose is higher, and the crystallization will be faster. Oppositely, honey with less glucose, relative to water, is a less saturated glucose solution and slow to crystallize [72,73,74][49][50][51]. Therefore, one of the critical properties of a solution in freezing is its tendency towards ice formation during cooling and warming. Fatemeh et al. (in 2019) had studied the thermal behavior of honey in embryo vitrification. In this study, sucrose is replaced by natural honey in a vitrified solution, which makes it more thermodynamically favorable and reduces the chance of ice crystal formation and cryodamage [22]. Due to the high cooling and warming rates, thermal stress brings unavoidable biological responses, including an excessive production of reactive oxygen species (ROS) [75][52]. ROS are believed to detrimentally affect mitochondrial activities, induce apoptosis, and decrease the synthesis of adenosine triphosphate (ATP) [76,77][53][54]. It has been previously shown that the promoted accumulation of ROS in heat-shocked embryos can be undermined, at least partially, by providing exogenous antioxidants, such as melatonin, ascorbic acid, and beta-mercaptoethanol [78,79,80][55][56][57].
2.3. Antioxidant Property
The consumption of antioxidants is a basic need of everyone who wishes to live a healthy life. Its major function in cells is to eliminate the harmful free radicals produced by common metabolic processes. These elements inhibit the destructive chemical reactions that cause food spoilage and many chronic illnesses. A. Gül & T. Pehlivan, R. Khalafi et al., and M. H. Roby et al. suggested that cancer and other chronic diseases could be prevented by consuming food containing an abundance of antioxidants. The major antioxidant present in honey includes amino acids (glutamine, glutamate, glycine, aspartic acid, and threonine), phenolic compounds (phenolic acids, tocopherol, quercetin, and flavonoids), vitamin C (galagin, pinobaxin, pinocembrin, and chrisin), and enzymes (catalase and glucose oxidase) [81,82,83][58][59][60]. Honey is becoming a popular source of antioxidants because antioxidant supply demand has widely increased in various applications. Genetic structure disruption and cellular damage occurred, due to oxidative stress, which is caused by the shortage of balancing the chemical reactions between the production of free radicals (ROS) and the natural protective effects of the body [84,85,86][61][62][63]. Honey phenol quercetin content directly binds to cells and strongly inhibits cellular transcription factor activities. The transcription factors inhibition improved the process of activation and phosphorylation, which avoids the cellular effect of the free radicals. It also decreases human fibrosarcoma protein expression levels and induces apoptosis of human osteosarcoma cells [87][64]. Honey has been used for a long time for medical needs, but its antioxidant property have recently come into the spotlight in cryopreservation [22]. Based on these observations, Fatemeh et al. (2019) investigated honey oxidative behavior as a cryoprotectant to improve embryo vitrification. Furthermore, it substantially reduced vitrified/warmed embryos ROS levels, due to its high antioxidant property [22].
3. Honey in Cryopreservation
Since early times, it has been observed that honey can be used to preserve and protect food and tissues by several means, most notably by its osmolality and antiseptic powers, which are provided by the hydrogen peroxide and phenol content (7). However, in the early 20th century, a new concept of preservation was developed, called cryopreservation. It studies low temperature (−196 °C) to preserve living cells and tissue. With the increasing interest in using less toxic/non-toxic chemicals in cryopreservation, as a substitute for the toxic freezing solution, researchers give rise to using natural products and avoiding chemicals. Thus far, the most studied and effective use of honey in cryopreservation was extended to facilitate fertility enhancement. Moreover, recent research has revealed that honey can also act as a non-permeating cryoprotectant, due to its ice inhibition and membrane-stabilizing effect. Figure 2 shows the general process of oocytes, embryos, and sperm preservation at −196 °C.
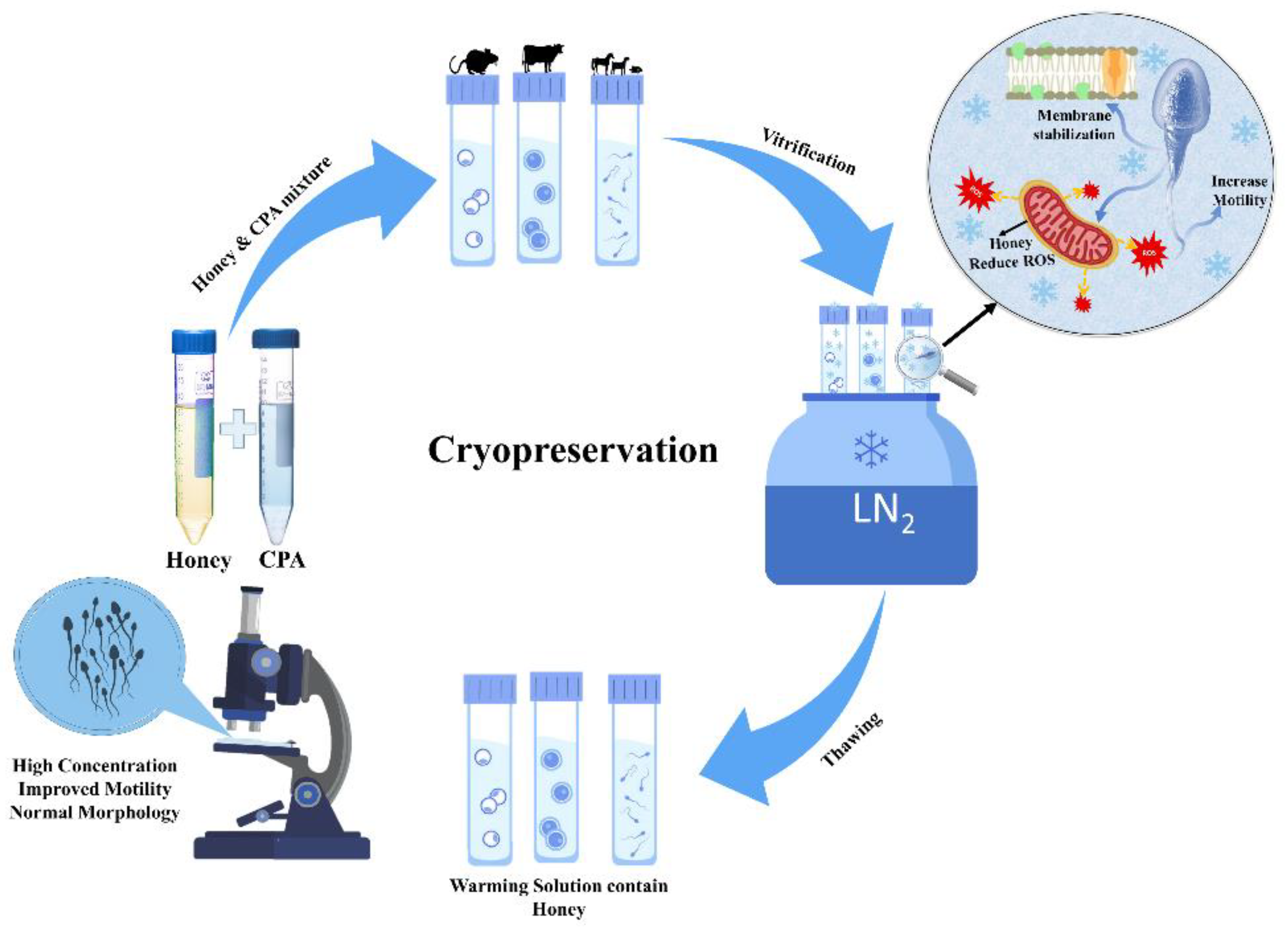

Figure 2.
Cryopreservation of fertility, using non-permeating cryoprotectant honey during vitrification.
3.1. Extenders
An extender is a medium to extend the volume of semen, through dilution, for artificial insemination, in order to maintain semen fertility in cryopreservation. In contrast, the addition of a freezing solution, called cryoprotectant, to the semen is used for extending the semen dilutions, which protect from cytotoxicity and osmotic stress during cryopreservation [134][65]. Several studies have been undertaken to use different materials and compounds, such as plant origins [135,136[66][67][68][69],137,138], whole milk, fish oil, and honey, to promote the quality of the extenders [139,140,141][70][71][72]. Historically, mammalian tissue fertility is enhanced by using honey in many cultures. Honey has the potential to protect the extracellular environment during cryopreservation because it contains a high amount of different sugars, which helps to increase the intracellular fluid efflux, thereby inhibiting ice crystal formation inside the cytoplasm of sperm [9,142][9][73]. Furthermore, a small amount of several antioxidant compounds are also contained in honey, including flavonoids, galagin, pinobaxin, and vitamin C [25,143][25][74].
Malik et al. discovered that the sperm motility (before freezing) and abnormality (after freezing and thawing) of semen are significantly affected by using honey to extenders. Adding honey to semen extenders noticeably improved sperm motility, acrosome integrity, membrane integrity, and viability index at 0 to 3 h of post-thawing [141][72]. This discovery was related to the results of other studies conducted on several species, respectively [25,144,145][25][75][76]. Other studies show that rosemary honey reduced DNA fragmentation, when combined with garlic and a skimmed milk-based extender [144][75]. The supplementation of 10% natural honey and cryoprotectant solution mixture in human semen caused a significant increase in the normal sperm morphology percentage [142,144][73][75]. Another experimental study, by El-Nattat et al., proved that different bull breed sperm quality was affected differently by adding various concentrations of honey in extenders. They suggested that 1% honey concentration, added to the extender (Bioxcell), could be more effective than Bioxcell without any additive in the cryopreservation of bull semen. For this reason, the Jersey bull showed the best quality of sperm, in comparison with other bull breeds [146,147,148][77][78][79]. Furthermore, El-Sheshtawy et al. (in 2016) proposed that using honey in the semen of Arab stallions, allowing for the inhibition of sperm DNA disorders, caused by oxidative stress, which protected sperm from cryoprotectant damage [21].
M. Hussain et al., J. Dorado et al., C.-H. Liu et al., and A. Ghaniei et al. have confirmed that using honey as an extender in cryopreservation improved the semen quality and acted as natural antibiotics against pathogenic bacteria. Moreover, it resolved many challenges, such as freezing solution toxicity, pH irregularity, ROS, energy source, damage of sperm membrane, and cryoshock preservatives [149,150,151,152][80][81][82][83].
3.2. Non-Permeating Natural Cryoprotectant
Honey is a supersaturated solution, and its unique composition provides several nutritional, pharmacological, and biological benefits to living cells, i.e., antioxidant, anti-inflammatory, antiproliferative, anticancer, antimicrobial, and antimetastatic activities [153][84]. It is a mixture of 25 sugars, mainly 75% monosaccharides, 10–15% disaccharides, and a minor amount of other sugars (i.e., rhamnose, erlose, trehalose, nigerobiose, sucrose, isomaltose, palatinose, maltose, maltotetraose, maltotriose, maltulose, melezitose, melibiose, raffinose, nigerose, etc.) found in honey [154,155,156,157][85][86][87][88]. It mainly consists of two kinds of sugar, fructose (38%) and glucose (31%) [31][48]. Besides sugars, many other bioactive substances are also present in honey, such as antioxidants, enzymes, vitamins, and organic acids [8,15,143][8][15][74].
Generally, sugars inhibit intracellular ice formation and prevent cell damage, due to the increasing the viscosity of the solution during vitrification, which raises the glass transition temperature to vitrify extracellular freezing solution [158][89]. For example, the glass transition temperature at −30 °C of sucrose solution, about 200 gL−1, is higher than 200 gL−1 of EG and glycerol solutions at −85 °C and −65 °C [159][90]. Sugars also act as plasma membrane stabilizing agents and protect the cell from freezing damage during cryopreservation [160][91]. Monosaccharides have a lower viscosity than disaccharides, which can be mixed more readily and efficiently, even in concentrated cryoprotectant solutions [161][92]. Disaccharides (i.e., sucrose, trehalose, and lactose) have been used in vitrification solutions as a non-permeant cryoprotectant, but commonly used disaccharides are sucrose and trehalose [162,163,164][93][94][95]. Raffinose (polysaccharide) has also proven to increase the survival rate of embryos after vitrification [163][94]. Fructose has a better effect on semen quality, as compared to disaccharides and polysaccharides, during the cryopreservation of red deer sperm [165][96]. Interestingly, using other disaccharides, such as sucrose and trehalose, not including lactose in dog sperm, reduces the acrosome injures and enhances the sperm viability, without any significant effect on motility after thawing, but fructose improved both the motility rates and acrosome injuries [166][97]. Other researchers proved that the effect of different sugars depends on their mass concentration, instead of their molar concentration during mouse sperm cryopreservation [167][98].
Furthermore, the supplementation of the two sugar (sucrose and glucose) mixture, instead of sucrose alone, in a vitrification solution, has more efficiently enhanced the vitrified bovine blastocysts survival rate [14]. Honey’s efficient properties, and its unique composition, make it ideal to use as natural non-permeating CPAs in cryopreservation. Table 1 summarizes the beneficial effect of using honey as a natural cryoprotectant in fertility cryopreservation. Honey decreases the effects of intracellular ice crystallization and the cytotoxic effects of CPA [168,169][99][100]. Honey is added to contribute to the vitrification medium viscosity and tonicity, which supports permeating CPAs in vitrification, which allows using lower concentrations of permeating CPAs, thus decreasing cytotoxic and osmotic shock effects permeating CPAs [170,171,172][101][102][103]. The honey-based media has proven to cause dehydration and rehydration in cells, sufficiently and safely, during vitrification [173,174][104][105]. In summary, the combination of honey and permeating CPAs in vitrification solution could improve mammalian tissues viability and functionalities after thawing, compared to vitrification media containing only permeating CPAs [175][106].
Table 1.
Selective studies for fertility cryopreservation using natural cryoprotectant honey.
| Natural CPA | Combination with Other CPAs | Cell or Tissue Type | Example of Species | Technique | Replacement Due to | Outcome | References |
|---|---|---|---|---|---|---|---|
| Natural honey | TCM-199 + EG + DMSO + CS | Bovine Oocytes | Cow | Vitrification | To investigate in vitro maturation (IVM), fertilization (IVF), and embryo development (IVC) of GV-stage oocytes vitrified in honey and sucrose solutions. | 1. Natural honey acted as a non-permeating CPA in vitrification solution. 2. Improved post-warm oocyte viability and embryonic development. 3. It shows better blastocyst development than sucrose (13% vs. 3%). |
Bilal Alfoteisy (2020) [27] |
| Natural honey | 7.5% EG + 7.5% DMSO | Embryo | Mouse | Vitrification | Replace sucrose with honey to reduce the chance of ice crystal formation and cryo damage. | Natural honey makes it more thermodynamically favorable by reducing the ROS level of vitrified embryos and decreasing the chances of cryodamage. | Fatemeh Sarmadia (2019) [22] |
| Natural honey | Nigella sativa | Sperm | Goat | Slow freezing | Compared it with a control group without any supplement. | The combination of honey and nigella sativa gives a better effect on post-thawed sperms than fresh sperms and prevents ice crystal formation. | Maidin (2018) [23] |
| Natural honey | N/A | Spermatozoa | Gourami | Slow freezing | To check the suitable concentration for gourami spermatozoa. | The combination of honey and DMSO gives the highest motility in comparison with the control group (0% honey solution). | Abinawanto (2017) [24] |
| Natural honey | DMSO | Semen (sperm motality) | Arabian Stallion | Slow freezing | To investigate the effect of different concentrations of natural honey on post-thawed sperm motility, viability index, membrane and acrosome integrities. |
Supplementation with honey (2%, 3%, and 4%) significantly improved post-thaw sperm motility, viability index. Additionally, it had a positive effect on membrane integrity and intact acrosome percentage at 0, 1, 2, 3, and 4h post-thawing. | Reda I. El-Sheshtawy (2016) [25] |
| Natural honey | Extender (mINRA-82 aliquots) | Sperm | African catfish | Slow freezing | To find out the cryopreservable effect of natural non-permeating cryoprotactent with frican catfish sperm, in comparison to DMSO. | A total of 10% honey allowed African catfish sperm to preserve into liquid nitrogen for 45 days. | Z.A. Muchlisin (2015) [26] |
3.3. Fertility Cryopreservation
Cryopreservation is a fundamental tool of assisted reproduction and establishing long-term banking germplasm. Fertility cryopreservation is challenging because of gametes and embryos morphological, functional, and genetic changes in the cells after freeze- thawing [176,177,178,179][107][108][109][110]. It gives several species, suffering from various life-threatening illnesses, a chance to conceive [180,181][111][112]. Furthermore, it maximizes reproductive material availability, facilitating reproductive procedures, independent of time and geographical location [163,182][94][113].
CPAs cytotoxicity is a fundamental limiting factor for the successful cryopreservation of cells or tissue in slow freezing and vitrification [183][114]. The most common approach to reduce toxicity has emerged, which entails combining various cryoprotectants, both permeating and non-permeating, thereby reducing individual concentrations and mitigating damage, while maintaining the overall protective effects [184,185][115][116]. The most commonly used permeating CPAs for fertility vitrification is EG and DMSO combination. EG is being established as the primary permeating CPA, due to its low toxicity [186,187][117][118]. However, cryoprotectants have some disadvantages, in that they can induce protein denaturation at higher temperatures and cause cryoprotectant toxicity in cellular systems [188][119]. The addition of non-permeating CPAs sugars in cryopreservation medium is one of the approaches to overcome the problems limiting the success of cell viability after warming [174][105].
Notably, different saccharides in non-permeating CPAs can have different protective behavior, due to their physical and chemical properties [48][120]. That leads to using several sugar combinations as non-permeating CPAs to decrease cryodamage [189][121]. Honey is a natural compound of multiple hydrocarbons that mainly consists of fructose and glucose. It is the most commonly used sugar to preserve germplasm [190][122]. Furthermore, the cryoprotective performance of honey alone, or in combination with other natural cryoprotectants, has been evaluated in fertility preservation. Table 1 summarizes the selected studies for fertility cryopreservation, using a medium containing natural non-permeating cryoprotectant honey.
A few recent experiments have been conducted to evaluate the fertility of frozen germplasm (i.e., sperm, ovaries, and embryos) with honey as a natural cryoprotectant. Alfoteisy et al. (2020) conducted an experiment on cow GV-stage oocytes in honey and sucrose vitrified solution to investigate in-vitro maturation (IVM), fertilization (IVF), and embryo development (IVC). A total of 1M concentration of natural non-permeating cryoprotectant honey is suitable for bovine oocyte vitrification, as compared with sucrose. The honey group achieved an improved blastocyst (13%), as compared with sucrose (3%), and can be used in vitrification solution to improve post-warm oocyte viability and embryonic development [27]. Another fertility trial with mouse embryo in 1 Osm/L honey with EG and DMSO (h-VS2) has been conducted by Sarmadia et al. (2019). It was shown that vitrified and warmed embryos, with h-VS2 solution, showed a significantly higher hatching rate, and the re-expansion rate of vitrified blastocyst was the same as the sucrose control group. The re-expansion rate will be reduced with further increase of the concentration. The re-expansion rates with h-VS2 and the sucrose control group are 94.6% and 97.9%. The hatching rate with h-VS2 obtained significantly higher rates than the control group, from 41.9% to 43.4% [22]. Based on this data, honey can reduce the chances of cryo-damage and ice crystal formation, due to its efficient thermal and antioxidant properties. Another important observation in this experiment is the potential capability of honey as an inhibitor to ROS, thus useful in embryo cryopreservation. Putri et al. (2020) evaluated that the best concentration of honey, as a natural and extracellular cryoprotectant, is 5% to maintain sperm motility for 48 h after cryopreservation, which gave the highest motility around 87.76%, in comparison with 0% honey (69.30%) [191][123]. Abinawanto et al. (2017) reported that the cryopreservation of gourami spermatozoa with 0.7% of the honey solution and 10% of DMSO cryoprotectant is highly effective, which gives the highest motility (80.78%). Based on cryoprotectant activities, honey is considered as a non-permeating or extracellular cryoprotectant, while DMSO is permeating or intracellular cryoprotectant [24]. Muchlisin et al. (2015) observed the cryoprotective effect of 10% honey, compared with DMSO, to find the suitable concentration for cryopreservation of siluriformes spermatozoa [26]. Furthermore, Maidin et al. (in 2018) demonstrated that goat semen treated with honey and nigella sativa oil has higher post-thawed motility (about 60.33% at 0 and 0.5 h). In comparison with the control without supplementation, the post-thawed mobility was 24.33%, which shows that the solution could protect sperm membrane and ice formation during cryopreservation, due to oxidative stress [23]. Sheshtawy et al. (in 2016) observed significant improvement in Arab stallions on post-thawed sperm motility, viability index, membrane, and acrosome integrities at 0, 1, 2 and 3 h. Therefore, fertility preservation with natural cryoprotectant honey may have longer viability, in combination and comparison with other cryoprotectants [192][124].
References
- Saba, Z.; Suzana, M.; My, Y.A. Honey: Food or medicine. Med. Health 2013, 8, 3–18.
- Bambang, N.; Ikhsan, M.; Sukri, N. Rheological Properties of Honey and its Application on Honey Flow Simulation through Vertical Tube. IOP Conf. Ser. Earth Environ. Sci. 2018, 334, 012041.
- Simsek, A.; Bilsel, M.; Goren, A.C. 13C/12C pattern of honey from Turkey and determination of adulteration in commercially available honey samples using EA-IRMS. Food Chem. 2012, 130, 1115–1121.
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC Trends Anal. Chem. 2017, 86, 25–38.
- Smanalieva, J.; Senge, B. Analytical and rheological investigations into selected unifloral German honey. Eur. Food Res. Technol. 2009, 229, 107–113.
- Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Karaman, S.; Dertli, E.; Sagdic, O.; Arici, M. Steady, dynamic and creep rheological analysis as a novel approach to detect honey adulteration by fructose and saccharose syrups: Correlations with HPLC-RID results. Food Res. Int. 2014, 64, 634–646.
- Shrestha, S.; Bhattarai, S.; Mahat, S.; Jha, M.; Amgain, K. Embalming–History to its Recent Advancements. Eur. J. Med. Sci. 2019, 1, 62–68.
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2010, 3, 15–23.
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. CryoLetters 2004, 25, 375–388.
- Nwosu, C.L. Cryopreservation of Plant Cells; Northwestern Oklahoma State University: Alva, OK, USA, 2015; Available online: https://www.academia.edu/download/37898406/CeeNonso_Seminar_-_Copy.pdf (accessed on 7 December 2021).
- Bhattacharya, M.S. A review on cryoprotectant and its modern implication in cryonics. Asian J. Pharm. 2016, 10, 154–159.
- Best, B.P. Cryoprotectant toxicity: Facts, issues, and questions. Rejuvenation Res. 2015, 18, 422–436.
- Cheepa, F.F.; Zhao, G.; Panhwar, F.; Memon, K. Controlled Release of Cryoprotectants by Near-Infrared Irradiation for Improved Cell Cryopreservation. ACS Biomater. Sci. Eng. 2021, 7, 2520–2529.
- Saito, N.; Imai, K.; Tomizawa, M. Effect of sugars-addition on the survival of vitrified bovine blastocysts produced in vitro. Theriogenology 1994, 41, 1053–1060.
- Pérez, R.A.; Iglesias, M.T.; Pueyo, E.; González, M.; de Lorenzo, C. Amino acid composition and antioxidant capacity of Spanish honeys. J. Agric. Food Chem. 2007, 55, 360–365.
- Bogdanov, S. Honey as nutrient and functional food. Proteins 2012, 1100, 1400–2700.
- Manyi-Loh, C.E.; Clarke, A.M.; Ndip, N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011, 5, 844–852.
- Makhloufi, C.; Kerkvliet, J.D.; D’albore, G.R.; Choukri, A.; Samar, R. Characterization of Algerian honeys by palynological and physico-chemical methods. Apidologie 2010, 41, 509–521.
- Brudzynski, K.; Miotto, D. The relationship between the content of Maillard reaction-like products and bioactivity of Canadian honeys. Food Chem. 2011, 124, 869–874.
- Abdel-Latif, M.M.; Windle, H.J.; Homasany, B.S.E.; Sabra, K.; Kelleher, D. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br. J. Pharmacol. 2005, 146, 1139–1147.
- El-Sheshtawy, R.I.; El-Badry, D.A.; Gamal, A.; El-Nattat, W.S.; Almaaty, A.M.A. Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters. Asian Pac. J. Reprod. 2016, 5, 331–334.
- Sarmadi, F.; Kazemi, P.; Tirgar, P.; Fayazi, S.; Esfandiari, S.; Sotoodeh, L.; Molaeian, S.; Dashtizad, M. Using natural honey as an anti-oxidant and thermodynamically efficient cryoprotectant in embryo vitrification. Cryobiology 2019, 91, 30–39.
- Maidin, M.S.; Padlan, M.; Azuan, S.; Jonit, R.; Mohammed, N.; Abdullah, R. Supplementation of Nigella sativa oil and honey prolong the survival rate of fresh and post-thawed goat sperms. Trop. Anim. Sci. J. 2018, 41, 94–99.
- Abinawanto, A.; Pratiwi, I.A.; Lestari, R. Sperm motility of giant gourami (Osphronemus goramy, Lacepede, 1801) at several concentrations of honey combined with DMSO after short-term storage. Aquac. Aquar. Conserv. Legis. 2017, 10, 156–163.
- El-Sheshtawy, R.I.; El-Nattat, W.S.; Sabra, H.A.; Ali, A.H. Effect of honey solution on semen preservability of local breeds of cattle bulls. World Appl. Sci. J. 2014, 32, 2076–2078.
- Za, M.; Wn, N.; Mn, S.A. Exploration of natural cryoprotectants for cryopreservation of African catfish, Clarias gariepinus, Burchell 1822 (Pisces: Clariidae) spermatozoa. Chezch J. Anim. Sci. 2015, 60, 10–15.
- Alfoteisy, B.; Singh, J.; Anzar, M. Natural honey acts as a nonpermeating cryoprotectant for promoting bovine oocyte vitrification. PLoS ONE 2020, 15, e0238573.
- Sopade, P.; Halley, P.; Bhandari, B.; D’arcy, B.; Doebler, C.; Caffin, N. Application of the Williams–Landel–Ferry model to the viscosity–temperature relationship of Australian honeys. J. Food Eng. 2003, 56, 67–75.
- Ahmed, J.; Prabhu, S.; Raghavan, G.; Ngadi, M. Physico-chemical, rheological, calorimetric and dielectric behavior of selected Indian honey. J. Food Eng. 2007, 79, 1207–1213.
- Juszczak, L.; Fortuna, T. Rheology of selected Polish honeys. J. Food Eng. 2006, 75, 43–49.
- Bakier, S. Influence of temperature and water content on the rheological properties of Polish honeys. Pol. J. Food Nutr. Sci. 2007, 57, 17–23.
- Gómez-Díaz, D.; Navaza, J.M.; Quintáns-Riveiro, L.C. Effect of temperature on the viscosity of honey. Int. J. Food Prop. 2009, 12, 396–404.
- Maldonado, G.E.; Navarro, A.S.; Yamul, D.K. A comparative study of texture and rheology of Argentinian honeys from two regions. J. Text. Stud. 2018, 49, 424–433.
- Al-Malah, K.I.; Abu-Jdayil, B.; Zaitoun, S.; Ghzawi, A.A.M. Application of WLF and Arrhenius kinetics to rheology of selected dark-colored honey. J. Food Process Eng. 2001, 24, 341–357.
- Recondo, M.; Elizalde, B.; Buera, M. Modeling temperature dependence of honey viscosity and of related supersaturated model carbohydrate systems. J. Food Eng. 2006, 77, 126–134.
- Bakier, S. Rheological properties of honey in a liquid and crystallized state. In Honey Analysis; IntechOpen: Vienna, Austria, 2017; pp. 115–137.
- Silva, V.M.d.; Torres Filho, R.d.A.; Resende, J.V.d. Rheological properties of selected Brazilian honeys as a function of temperature and soluble solid concentration. Int. J. Food Prop. 2017, 20, S2481–S2494.
- Bhandari, B.; D’Arcy, B.; Chow, S. Rheology of selected Australian honeys. J. Food Eng. 1999, 41, 65–68.
- Lazaridou, A.; Biliaderis, C.G.; Bacandritsos, N.; Sabatini, A.G. Composition, thermal and rheological behaviour of selected Greek honeys. J. Food Eng. 2004, 64, 9–21.
- Zaitoun, S.; Ghzawi, A.A.-M.; Al-Malah, K.I.; Abu-Jdayil, B. Rheological properties of selected light colored Jordanian honey. Int. J. Food Prop. 2001, 4, 139–148.
- Gómez-Díaz, D.; Navaza, J.M.; Quintáns-Riveiro, L.C. Rheological behaviour of Galician honeys. Eur. Food Res. Technol. 2006, 222, 439–442.
- Mossel, B.; Bhandari, B.; D’Arcy, B.; Caffin, N. Use of an Arrhenius model to predict rheological behaviour in some Australian honeys. LWT-Food Sci. Technol. 2000, 33, 545–552.
- Ahmed, J. Advances in rheological measurements of food products. Curr. Opin. Food Sci. 2018, 23, 127–132.
- Ahmed, J.; Ptaszek, P.; Basu, S. Food rheology: Scientific development and importance to food industry. In Advances in Food Rheology and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–4.
- Karasu, S.; Toker, O.S.; Yilmaz, M.T.; Karaman, S.; Dertli, E. Thermal loop test to determine structural changes and thermal stability of creamed honey: Rheological characterization. J. Food Eng. 2015, 150, 90–98.
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. Effect of moisture content on the viscosity of honey at different temperatures. J. Food Eng. 2006, 72, 372–377.
- Asoiro, F.U.; Simeon, M.I.; Ohagwu, C.J.; Abada, U.C. Evaluation of the physicochemical and thermal properties of honey samples from different floral locations in Enugu North senatorial zone, Nigeria. In Proceedings of the 12th CIGR Section VI International Symposium, Ibadan, Nigeria, 22–25 October 2018; pp. 28–41.
- M Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638.
- Venir, E.; Spaziani, M.; Maltini, E. Crystallization in “Tarassaco” Italian honey studied by DSC. Food Chem. 2010, 122, 410–415.
- Ouchemoukh, S.; Schweitzer, P.; Bey, M.B.; Djoudad-Kadji, H.; Louaileche, H. HPLC sugar profiles of Algerian honeys. Food Chem. 2010, 121, 561–568.
- Dobre, I.; Georgescu, L.A.; Alexe, P.; Escuredo, O.; Seijo, M.C. Rheological behavior of different honey types from Romania. Food Res. Int. 2012, 49, 126–132.
- Mehaisen, G.M.; Saeed, A.M.; Gad, A.; Abass, A.O.; Arafa, M.; El-Sayed, A. Antioxidant capacity of melatonin on preimplantation development of fresh and vitrified rabbit embryos: Morphological and molecular aspects. PLoS ONE 2015, 10, e0139814.
- Herrick, J.R.; Wang, C.; Machaty, Z. The effects of permeating cryoprotectants on intracellular free-calcium concentrations and developmental potential of in vitro-matured feline oocytes. Reprod. Fertil. Dev. 2016, 28, 599–607.
- Nohales-Córcoles, M.; Sevillano-Almerich, G.; Di Emidio, G.; Tatone, C.; Cobo, A.; Dumollard, R.; De Los Santos Molina, M. Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum. Reprod. 2016, 31, 1850–1858.
- Castillo-Martín, M.; Bonet, S.; Morató, R.; Yeste, M. Comparative effects of adding β-mercaptoethanol or L-ascorbic acid to culture or vitrification–warming media on IVF porcine embryos. Reprod. Fertil. Dev. 2014, 26, 875–882.
- Gupta, M.K.; Uhm, S.J.; Lee, H.T. Effect of vitrification and beta-mercaptoethanol on reactive oxygen species activity and in vitro development of oocytes vitrified before or after in vitro fertilization. Fertil. Steril. 2010, 93, 2602–2607.
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141.
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065.
- Khalafi, R.; Goli, S.A.H.; Behjatian, M. Characterization and classification of several monofloral Iranian honeys based on physicochemical properties and antioxidant activity. Int. J. Food Prop. 2016, 19, 1065–1079.
- Roby, M.H.; Abdelaliem, Y.F.; Esmail, A.-H.M.; Mohdaly, A.A.; Ramadan, M.F. Evaluation of Egyptian honeys and their floral origins: Phenolic compounds, antioxidant activities, and antimicrobial characteristics. Environ. Sci. Pollut. Res. 2020, 27, 20748–20756.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Grune, T. Free Radicals and Diseases: Gene Expression, Cellular Metabolism and Pathophysiology; IOS Press: Amsterdam, The Netherlands, 2005; Volume 367.
- Poli, G. Free Radicals in Brain Pathophysiology; CRC Press: Boca Raton, FL, USA, 2000.
- Milner, J.A.; Romagnolo, D.F.; Connor, J.; Lee, S. Bioactive Compounds and Cancer; Springer, Humana Press: New York, NY, USA, 2010.
- Muchlisin, Z.A. Current status of extenders and cryoprotectants on fish spermatozoa cryopreservation. Biodiversitas J. Biol. Divers. 2005, 6, 66–69.
- Layek, S.; Mohanty, T.; Kumaresan, A.; Parks, J. Cryopreservation of bull semen: Evolution from egg yolk based to soybean based extenders. Anim. Reprod. Sci. 2016, 172, 1–9.
- Al-Daraji, H.J. Effect of adding orange juice into semen diluents on quality and storage ability of cocks’ semen. Res. Opin. Anim. Vet. Sci. 2012, 2, 485–489.
- Gunawan, M.; Setiorini, S.; Fitri, H.; Kaiin, E. The effect of siam orange juice (Citrus nobilis Lour.) in extender on Garut Ram (Ovis aries L.) spermatozoa quality post-cryopreservation. J. Phys. Conf. Ser. 2020, 1442, 012068.
- Adekunle, E.O.; Daramola, J.O.; Sowande, O.S.; Abiona, J.A.; Abioja, M.O. Effects of apple and orange juices on quality of refrigerated goat semen. J. Agric. Sci. Belgrade 2018, 63, 53–65.
- Khoshvaght, A.; Towhidi, A.; Zare-Shahneh, A.; Noruozi, M.; Zhandi, M.; Davachi, N.D.; Karimi, R. Dietary n-3 PUFAs improve fresh and post-thaw semen quality in Holstein bulls via alteration of sperm fatty acid composition. Theriogenology 2016, 85, 807–812.
- Amin, B.Y.; Prasad, J.K.; Ghosh, S.K.; Lone, S.A.; Kumar, A.; Mustapha, A.R.; Din, O.; Kumar, A. Effect of various levels of dissolved oxygen on reactive oxygen species and cryocapacitation-like changes in bull sperm. Reprod. Domest. Anim. 2018, 53, 1033–1040.
- Malik, A. Effects of honey supplementation into the extender on the motility, abnormality and viability of frozen thawed of Bali bull spermatozoa. Asian J. Anim. Vet. Adv. 2019, 13, 109–113.
- Fakhrildin, M.-B.M.; Alsaadi, R.A. Honey Supplementation to semen-freezing medium improveshuman sperm parameters post-thawing. J. Fam. Reprod. Health 2014, 8, 27–31.
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689.
- Jerez-Ebensperger, R.; Luno, V.; Olaciregui, M.; Gonzalez, N.; de Blas, I.; Gil, L. Effect of pasteurized egg yolk and rosemary honey supplementation on quality of cryopreserved ram semen. Small Rumin. Res. 2015, 130, 153–156.
- Ögretmen, F.; İnanan, B.E. Evaluation of cryoprotective effect ofturkish pine honey on common carp (Cyprinus Carpio) Spermatozoa. CryoLetters 2014, 35, 427–437.
- Chung, E.; Nayan, N.; Nasir, N.; Hing, P.; Ramli, S.; Rahman, M.; Kamalludin, M. Effect of honey as an additive for cryopreservation on bull semen quality from different cattle breeds under tropical condition. J. Anim. Health Prod. 2019, 7, 171–178.
- Yimer, N.; Muhammad, N.; Sarsaifi, K.; Rosnina, Y.; Wahid, H.; Khumran, A.; Kaka, A. Effect of honey supplementation into Tris Extender on Cryopreservation of Bull Spermatozoa. Malays. J. Anim. Sci. 2015, 18, 47–54.
- Zaghloul, A. Relevance of honey bee in semen extender on the quality of chilled-stored ram semen. J. Anim. Poult. Prod. 2017, 8, 1–5.
- Hussain, M.; Begum, S.S.; Kalita, M.K.; Ahmed, K.U.; Nath, R. Additives used in semen preservation in animals: A short review. Int. J. Chem. Stud. 2018, 6, 354–361.
- Dorado, J.; Hidalgo, M.; Acha, D.; Ortiz, I.; Bottrel, M.; Azcona, F.; Carrasco, J.J.; Gómez-Arrones, V.; Demyda-Peyrás, S. Cryopreservation of Andalusian donkey (Equus asinus) spermatozoa: Use of alternative energy sources in the freezing extender affects post-thaw sperm motility patterns but not DNA stability. Anim. Reprod. Sci. 2019, 208, 106126.
- Liu, C.-H.; Dong, H.-B.; Ma, D.-L.; Li, Y.-W.; Han, D.; Luo, M.-J.; Chang, Z.-L.; Tan, J.-H. Effects of pH during liquid storage of goat semen on sperm viability and fertilizing potential. Anim. Reprod. Sci. 2016, 164, 47–56.
- Ghaniei, A.; Eslami, M.; Zadeh Hashem, E.; Rezapour, R.; Talebi, A. Quercetin attenuates H2O2-induced toxicity of rooster semen during liquid storage at 4 °C. J. Anim. Physiol. Anim. Nutr. 2019, 103, 713–722.
- Calixto-Campos, C.s.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808.
- Cummings, J.; Stephen, A. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18.
- Kamal, M.A.; Klein, P. Determination of sugars in honey by liquid chromatography. Saudi J. Biol. Sci. 2011, 18, 17–21.
- Jaganathan, S.K. Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. Sci. World J. 2012, 2012, 372345.
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323.
- Amorim, C.A.; Curaba, M.; Van Langendonckt, A.; Dolmans, M.-M.; Donnez, J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod. Biomed. Online 2011, 23, 160–186.
- Levine, H.; Slade, L. Thermomechanical properties of small-carbohydrate–water glasses and ‘rubbers’. Kinetically metastable systems at sub-zero temperatures. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 2619–2633.
- Quan, G.B.; Hong, Q.H.; Lan, Z.G.; Yang, H.Y.; Wu, S.S. Comparison of the effect of various disaccharides on frozen goat spermatozoa. Biopreserv. Biobank. 2012, 10, 439–445.
- McWilliams, R.; Gibbons, W.; Leibo, S. Fertilization and early embryology: Osmotic and physiological responses of mouse zygotes and human oocytes to mono-and disaccharides. Hum. Reprod. 1995, 10, 1163–1171.
- Mazni, O.A.; Valdez, C.; Takahashi, Y.; Hishinuma, M.; Kanagawa, H. Quick freezing of mouse embryos using ethylene glycol with lactose or sucrose. Anim. Reprod. Sci. 1990, 22, 161–169.
- Kuleshova, L.; Macfarlane, D.R.; Trounson, A.O.; Shaw, J.M. Sugars exert a major influence on the vitrification properties of ethylene glycol-based solutions and have low toxicity to embryos and oocytes. Cryobiology 1999, 38, 119–130.
- Saha, S.; Rajamahendran, R.; Boediono, A.; Sumantri, C.; Suzuki, T. Viability of bovine blastocysts obtained after 7, 8 or 9 days of culture in vitro following vitrification and one-step rehydration. Theriogenology 1996, 46, 331–343.
- Fernández-Santos, M.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.; Soler, A.; De Paz, P.; Anel, L.; Garde, J. Extender osmolality and sugar supplementation exert a complex effect on the cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa. Theriogenology 2007, 67, 738–753.
- Yildiz, C.; Kaya, A.; Aksoy, M.; Tekeli, T. Influence of sugar supplementation of the extender on motility, viability and acrosomal integrity of dog spermatozoa during freezing. Theriogenology 2000, 54, 579–585.
- Koshimoto, C.; Mazur, P. The effect of the osmolality of sugar-containing media, the type of sugar, and the mass and molar concentration of sugar on the survival of frozen-thawed mouse sperm. Cryobiology 2002, 45, 80–90.
- Takeya, S.; Hori, A.; Hondoh, T.; Uchida, T. Freezing-memory effect of water on nucleation of CO2 hydrate crystals. J. Phys. Chem. B 2000, 104, 4164–4168.
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423.
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19.
- Mazur, P.; Seki, S.; Pinn, I.L.; Kleinhans, F.; Edashige, K. Extra-and intracellular ice formation in mouse oocytes. Cryobiology 2005, 51, 29–53.
- Papis, K.; Shimizu, M.; Izaike, Y. Factors affecting the survivability of bovine oocytes vitrified in droplets. Theriogenology 2000, 54, 651–658.
- Han, X.; Critser, J.K. Measurement of the size of intracellular ice crystals in mouse oocytes using a melting point depression method and the influence of intracellular solute concentrations. Cryobiology 2009, 59, 302–307.
- Leibo, S. A one-step method for direct nonsurgical transfer of frozen-thawed bovine embryos. Theriogenology 1984, 21, 767–790.
- Huang, J.; Li, Q.; Zhao, R.; Li, W.; Han, Z.; Chen, X.; Xiao, B.; Wu, S.; Jiang, Z.; Hu, J. Effect of sugars on maturation rate of vitrified-thawed immature porcine oocytes. Anim. Reprod. Sci. 2008, 106, 25–35.
- Massip, A. Cryopreservation of bovine oocytes: Current status and recent developments. Reprod. Nutr. Dev. 2003, 43, 325–330.
- Bogliolo, L.; Ariu, F.; Fois, S.; Rosati, I.; Zedda, M.T.; Leoni, G.; Succu, S.; Pau, S.; Ledda, S. Morphological and biochemical analysis of immature ovine oocytes vitrified with or without cumulus cells. Theriogenology 2007, 68, 1138–1149.
- Rojas, C.; Palomo, M.J.; Albarracín, J.L.; Mogas, T. Vitrification of immature and in vitro matured pig oocytes: Study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology 2004, 49, 211–220.
- Estudillo, E.; Jiménez, A.; Bustamante-Nieves, P.E.; Palacios-Reyes, C.; Velasco, I.; López-Ornelas, A. Cryopreservation of Gametes and Embryos and Their Molecular Changes. Int. J. Mol. Sci. 2021, 22, 10864.
- Mazur, P. Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys. 1990, 17, 53–92.
- Mazur, P. Principles of cryobiology. In Life in the Frozen State; CRC Press: Boca Raton, FL, USA, 2004; pp. 29–92.
- Vincent, C.; Turner, K.; Pickering, S.; Johnson, M. Zona pellucida modifications in the mouse in the absence of oocyte activation. Mol. Reprod. Dev. 1991, 28, 394–404.
- Fahy, G.M. Cryoprotectant toxicity neutralization. Cryobiology 2010, 60, S45–S53.
- Rall, W.; Wood, M. High in vitro and in vivo survival of day 3 mouse embryos vitrified or frozen in a non-toxic solution of glycerol and albumin. Reproduction 1994, 101, 681–688.
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature 1985, 313, 573–575.
- Jomha, N.M.; Weiss, A.D.; Forbes, J.F.; Law, G.K.; Elliott, J.A.; McGann, L.E. Cryoprotectant agent toxicity in porcine articular chondrocytes. Cryobiology 2010, 61, 297–302.
- Ali, J.; Shelton, J. Design of vitrification solutions for the cryopreservation of embryos. Reproduction 1993, 99, 471–477.
- Chao, N. Fish sperm cryopreservation in Taiwan: Technology advancement and extension efforts. B. Inst. Zo. Acad. Sin. Monogr. 1991, 16, 263–283.
- Abdel-Aal, E.M.; Ziena, H.; Youssef, M. Adulteration of honey with high-fructose corn syrup: Detection by different methods. Food Chem. 1993, 48, 209–212.
- Pereira, C.S.; Hünenberger, P.H. Interaction of the sugars trehalose, maltose and glucose with a phospholipid bilayer: A comparative molecular dynamics study. J. Phys. Chem. B 2006, 110, 15572–15581.
- Lazarević, K.B.; Andrić, F.; Trifković, J.; Tešić, Ž.; Milojković-Opsenica, D. Characterisation of Serbian unifloral honeys according to their physicochemical parameters. Food Chem. 2012, 132, 2060–2064.
- Putri, N.M.; Kreshanti, P.; Tunjung, N.; Indania, A.; Basuki, A.; Sukasah, C.L. Efficacy of honey dressing versus hydrogel dressing for wound healing. AIP Conf. Proc. 2021, 2344, 020022.
- Elkhawagah, A.R.M. Effect of honey supplementation on Egyptian buffalo semen. Anim. Reprod. 2018, 14, 1103–1109.
More
