Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jianxun Ding and Version 2 by Jessie Wu.
In recent decades, the biomedical applications of mesenchymal stem cells (MSCs) have attracted increasing attention. MSCs are easily extracted from the bone marrow, fat, and synovium, and differentiate into various cell lineages according to the requirements of specific biomedical applications. As MSCs do not express significant histocompatibility complexes and immune stimulating molecules, they are not detected by immune surveillance and do not lead to graft rejection after transplantation. These properties make them competent biomedical candidates, especially in tissue engineering.
- mesenchymal stem cell
- extraction
- cell differentiation
- growth factor
- scaffold
- biomaterial
- tissue engineering
- regenerative medicine
- biomedical application
1. Introduction
Since the discovery of spindle-shaped, bone marrow-derived plastic-adherent cells in the mid-1970s [1], science has come a long way, and studies have found that these cells could differentiate into osteoblasts and chondrocytes [2][3][2,3]. Techniques for extraction, culture, and induction of mesenchymal stem cells (MSCs) have improved, with almost all MSC types derived from various tissues now capable of differentiation into osteocytes and end-stage lineages [4]. The rapid development of molecular biology and transplantation techniques has benefitted MSC applications in regenerative medicine.
MSCs are an ideal cell source for tissue regeneration, owing to the excellent properties as follows. MSCs exist in almost all tissues, including bone marrow, adipose, and synovium [5], and are easily extracted. MSCs can differentiate into almost any end-stage lineage cells to enable their seeding in specific scaffolds (Figure 1) [6]. Their immunological properties, including anti-inflammatory, immunoregulatory, and immunosuppressive capacities, contribute to their potential role as immune tolerant agents [7][8][7,8].
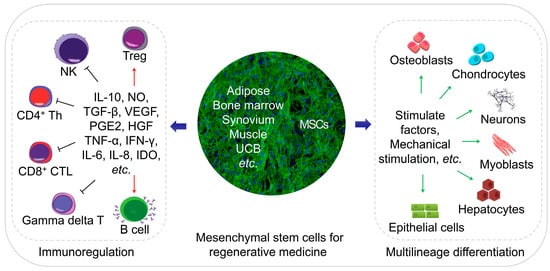
Figure 1. Schematic diagram of regenerative medicine based on mesenchymal stem cells (MSCs). The MSCs can be easily extracted from varies tissues, and the multilineage differentiation and immunoregulatory properties of MSCs make them an ideal cell therapeutic candidate.
Numerous studies have explored MSCs for tissue regeneration in several animal models in vitro; trials have not been limited to preclinical validation. Several clinical reports verify the potential efficacy of MSC-based cell therapy; although its effectiveness remains limited, the outcomes are inspiring.
2. Bone Regeneration
Bone defects frequently accompany recovery from trauma, revision arthroplasty, or tumor resection surgeries. Autologous bone grafting represents the gold standard therapeutic strategy, despite its many drawbacks, including (1) the limited supply of autologous bone, (2) increased operation time and blood loss, (3) temporary disruption of bone structure in the donor site, and (4) donor site morbidity [9][51]. Allografting carries a risk of disease and/or infection [10][52]. Therefore, MSC-based bone regeneration is considered an optimal approach [11][53].
The MSC osteoblast-differentiation capacity has been identified [2][3][2,3], with BMSCs representing the most frequently applied cells for osteoblast differentiation [2]. Comparative studies evaluating the osteogenic ability of other MSC types yielded no definitive conclusions. By contrast, UCB-MSCs show better angiogenic capacity, supporting more abundant blood supply during bone regeneration [12][54], which promotes rapid tissue reconstruction. In addition to BMSCs, human dental pulp stem cells (hDPSCs) show excellent vascular differentiation potential while differentiating into osteoblasts, which subsequently support bone regeneration [13][55]. However, these hDPSCs are screened from a stromal vascular dental pulp fraction; therefore, this represents a limited source for further research and application. Since ADSCs can be routinely isolated from lipoaspirate with a high degree of purity with minimal donor site morbidity or patient discomfort, ADSCs are considered to have the most significant potential as a primary source for clinical bone tissue engineering [14][15][16][48,56,57]. Additional comparative and screening studies are necessary to identify other cell sources with applications in bone reconstruction.
Stimulating factors play an important role in directing MSC differentiation into target cells in vitro. The most commonly used inducing factor for osteogenesis is the bone morphogenetic protein-2 (BMP-2), which is usually immobilized on scaffolds to promote osteoblast differentiation. BMP-2 exhibits a strong osteogenic ability, which can be tested by the osteoblast activity and/or expression of bone markers, such as alkaline phosphatase (ALP), osteopontin (OPN), and osteocalcin (OCN) [17][18][19][58,59,60]. BMP-7 activates the transforming growth factor-β (TGF-β) /SMAD signaling in CD105+ MSCs to enhance the expression of osteogenesis-related genes [20][61]; Wnt11 enhances the osteogenic potential of BMP-9 [21][62]. Nano-hydroxyapatite [17][58] and strontium [22][63] are used as osteogenic regulators in tissue engineering to promote osteogenic differentiation of MSCs while changing the physical properties of the scaffolds.
Studies of MSC-based cell therapy for bone defects and the use of novel scaffolds describe inspiring advances in vitro and in vivo [23][24][64,65]. Clinical applications of MSCs in bone reconstruction have been described, including those involving implantation of scaffolds seeded with MSCs into bone defect sites. Specifically, dentists have used this technique to address alveolar cleft defects, jaw defect reconstruction, and maxillary sinus augmentation, with excellent outcomes [25][26][27][66,67,68]. Defects in or non-union of human tubular bone have been tentatively treated via local implantation of MSCs with or without scaffolds [28][29][69,70].
3. Cartilage Repair
Cartilage defect repair is one of the significant challenges faced by orthopedic surgeons. Due to the inherent avascular nature of cartilage and the proliferation of mature chondrocytes, cartilage is greatly limited in its ability to repair itself. Currently, the clinically applied cartilage repair techniques, such as bone marrow stimulation and osteochondral transplantation, have their limitations. Fibrocartilage produced by bone marrow stimulation is not strong enough, and grafts for osteochondral transplantation are challenging to integrate.
MSCs offer a new strategy for the repair of damaged cartilage, as they can differentiate into chondrocytes [2][3][2,3]. An integrated cartilage reconstruction unit comprises cells, scaffolds, and stimulatory factors, with BMSCs [30][71], ADSCs [15][56], and SMSCs [31][72], used as the primary cell sources. Among these, BMSCs displayed better chondrogenic capacity in vitro and in vivo [32][33][30][33,34,71], although SMSCs show better proliferation and differentiation potential and less hypertrophy than BMSCs and ADSCs [31][72]. Cartilage reconstruction requires a combination of multiple stimulating factors; co-culture of chondrocytes and MSCs would achieve better results than the application of MSCs alone [34][73].
Novel bioactive three-dimensional (3D) scaffolds, such as hydrogels [13][55] and electrospun scaffolds [35][74], have undergone constant improvement. These scaffolds provide an optimal 3D microenvironment for cartilage regeneration. Moreover, hydrogels can regulate MSC proliferation and differentiation due to their high water content and biocompatibility and similar properties to the extracellular matrix (ECM) [13][55]. Injectable hydrogels enable minimally invasive treatment of large areas of cartilage defects [36][75]; thus, hydrogels loaded with MSCs and stimulating factors are highly efficacious for the repair of cartilage damage. The development of electrospun scaffolds suggests that the arrangement of nanofibers also affects cell differentiation and provides a different approach to cartilage repair [35][74]. Stimulating factors are necessary for cartilage engineering and responsible for inducing, accelerating, and/or enhancing cartilage formation. Common stimulating factors include BMP-2/-4, insulin-like growth factor (IGF)-1, and TGF-β1/-β3 [37][38][39][76,77,78]. Moreover, physical stimuli, such as hydrostatic pressure and dynamic compression, have been explored to induce MSC-mediated cartilage formation [40][79].
The first preclinical trial of MSC application for cartilage repair occurred in 1994 [41][80]. MSCs were seeded into a collagen (Col) gel to treat a full-thickness defect in rabbit femoral cartilage, resulting in better outcomes than those observed in a control group. This defect model was subsequently used as a classical cartilage defect model for cartilage regeneration; subsequently, numerous trials have been conducted in both animal models and humans to evaluate MSC-based therapy for cartilage damage [42][81].
Despite clinical trials being conducted, there are no commercially available products for MSC-based cartilage reconstruction [43][44][82,83]. Several studies investigated the effects of expanded MSCs in vivo on damage to human articular cartilage. Transplantation of expanded autologous BMSCs improved cartilage quality in patients with chronic knee osteoarthritis [44][83], although the clinical improvement was not significant [43][82]. Other studies reported the injection of allogeneic MSCs into joints in the presence or absence of pre-mixing with autologous chondrocytes [42][81]. All the clinical outcomes indicated the safety of these therapeutic approaches, and their ability to relieve some symptoms, although their ability to repair the effects of cartilage damage was not always apparent. MSC transplantation showed better results for early lesions [42][81].
There remain many challenges for MSC-based cartilage regeneration, including the identification of optimal cell sources. Additional studies are needed to enable the use of MSC-based materials as commercial products for implantation to promote cartilage regeneration.
4. Regeneration of Other Musculoskeletal Tissues
Recent studies investigated the MSC-mediated regeneration of musculoskeletal tissues outside the bone and cartilage, including the meniscus, tendons and ligaments, and intervertebral discs (IVD).
Meniscus regeneration has received increasing attention. Intra-articular administration of MSCs to promote meniscal regeneration was first performed with favorable outcomes [45][84]. Similar to its use for cartilage regeneration, hydrogels [46][85] and electrospun scaffolds [47][86] loaded with MSCs were used to reconstruct the meniscus. Moreover, the meniscus-derived decellularized matrix shows better histocompatibility and is more capable of inducing MSC differentiation as compared with natural or synthetic polymer materials [48][87]. Scaffold-free tissue-engineered constructs show promise as an MSC-based implantation technique to repair meniscal lesions [49][88]. Tarafder et al. [50][89] proposed the recruitment of synovial MSCs through connective tissue TGF and TGF-β3 to repair meniscus injury, thereby avoiding the disadvantages of cell-based techniques. Mechanical stimulation is crucial for meniscus growth and maintenance, with mechanical stimuli, such as dynamic compression and tensile loads applied for meniscus repair [51][90]. Although satisfactory results were obtained in animal models, there remains a lack of evidence in humans regarding the capability of MSCs for forming durable tissues similar to the meniscus [52][91].
Tendon injury is a common problem associated with sports [53][92]. BMP-14 induces myogenic differentiation of BMSCs via the sirtuin-1−Janus N-terminal kinase (JNK)/SMAD1-peroxisome proliferator-activated receptor-γ signaling pathway [54][93]. Studies describing tendogenic differentiation of MSCs were not limited to stimulating factors [55][94] and scaffolds [56][95] but also referred to mechanical stimuli that play essential roles in MSC differentiation into tendon lineages. Uniaxial cyclic stretching promoted tendogenic differentiation of MSCs in vitro and in vivo [57][96]; however, MSCs did not repair tendon injury but only delayed lesion progression [58][97].
With the increasing age of populations, IVD degeneration has become prevalent. MSCs represent promising candidates for disc regeneration; scaffolds made of Col provide readily available support for chondrogenic differentiation of MSCs in vitro, although the phenotype of the differentiated MSCs is not yet equivalent to that of nucleus pulposus (NP) cells [59][98]. The acellular matrices derived from NP cells stimulated by TGF-β3 also enhance MSC differentiation [60][99]. Transfection of adenoviral expression of SOX-9 and BMP-2 in BMSCs increased Col II and aggrecan expression, and promoted IVD repair [61][100]. Varma et al. [62][101] used a hydrogel loaded with two different concentrations of MSCs to repair NP, and showed that MSC inoculation at a lower density resulted in a better NP-specific matrix phenotype. A systematic review of MSC-based cell therapy for IVD indicated the safety and effectiveness of short-term MSC transplantation, as well as the necessity for human-based clinical trials [63][102]. In 2011, expanded autologous BMSCs injected into patients with lumbar disc degeneration revealed several advantages and better prognosis relative to the current gold standard treatments [64][103]. Clinical percutaneous injection of autologous bone marrow concentrate cells into a patient with degenerative IVD resulted in decreased lumbar discogenic pain within 13 years [65][104]. Clinical studies [66][105] indicated that MSC transplantation represents a safe treatment option for degenerative IVD; the specific effects need verification by additional clinical trials.
5. Central Nervous System Rebuilding
The adult central nervous system (CNS) lacks the ability to repair damaged neurons, so the damage of CNS is irreversible, and there is currently no effective repair method for CNS injury in clinical practice repair. In the area of CNS regeneration, MSC-based therapy mainly focuses on two areas: Damage or injury of the CNS caused by severe trauma and continuous ischemia and CNS dysfunction caused by neurologic diseases. To date, BMSCs and ADSCs are the most extensively studied cell sources for CNS repair, with each showing similar neuronal differentiation potential [67][68][35,36]. BMSCs can reduce scar formation around spinal cord injury (SCI) lesions and promoted axonal regeneration [69][106]; however, ADSCs might represent a more suitable cell source owing to their easy extraction and abundant sources. ADSCs inhibit inflammation of the nervous system and improve the recovery of function from traumatic brain injury via neural stem cells [70][107]. UCB-MSCs can be induced to differentiate into neuron-like cells in vitro [71][37]; DPSCs can differentiate into neurons and express multiple factors that promote neuronal and axonal regeneration [72][108].
MSC expression of neuronal or astrocytic markers has been observed in vitro [73][109] and in vivo [74][110]. To promote MSC-based CNS restoration, gene-modified MSCs, such as neurotrophin-3-transferred BMSCs, showed improved neuronal differentiation in vivo [75][111]. Persistent release of specific cytokines and growth factors, which can facilitate neurogenesis, angiogenesis, and synaptogenesis, creates a favorable microenvironment for angiogenesis or remyelination during reconstruction [76][112]. IGF-1-transfected spinal cord-derived neural stem cells displayed higher viability and the ability to differentiate into oligodendrocytes [77][113]. Moreover, MSCs can induce T cell tolerance and release of paracrine anti-inflammatory factors, such as TGF-β, that promote neuroprotective effects [78][114].
Animal models of traumatic and ischemic brain injury or SCI have been used to evaluate MSC-based therapy [79][80][115,116]. A meta-analysis of 1568 rats with traumatic SCI showed that MSC therapy provided a substantial beneficial effect on locomotor recovery [80][116]. Clinical studies indicated MSC-based therapy as a safe and feasible technique for patients with SCI and/or traumatic brain injury [81][82][117,118]. Migration of MSC pretreated under hypoxic conditions to the peri-cerebral injury area of cerebral hemorrhagic stroke victimized rats resulted in the release of various growth factors to promote neurogenesis and neurological recovery [83][119]. For neurological diseases, non-expanded or expanded MSCs have been widely used for the treatment of multiple sclerosis [84][120], amyotrophic lateral sclerosis [85][121], ischemic stroke [86][122], and Parkinson’s disease [87][123]. Most of the beneficial effects of MSCs on neurological diseases are associated with their immunomodulatory and neuroprotective properties exerted following local injection of non-expanded or expanded autologous MSCs, with clinical trials assessing their ability to achieve promising outcomes and different degrees of remission.
6. Peripheral Nervous System Rebuilding
Peripheral nervous system (PNS) injury is mainly caused by severe trauma, usually accompanied by bone fracture and vascular damage. Autologous nerve grafting (autologous nerve bridging) is the gold standard for peripheral nerve repair; however, limited donor nerve resources and other issues preclude the search for new therapeutic strategies. Schwann cells, neurotrophic factors, and anti-inflammatory cells work together to promote peripheral nerve regeneration, with this process involving axonal sprouting and fiber myelination.
There are few comparative studies concerning the effects of different MSC types on animal models of peripheral nerve injury. ADSCs are more suitable cell sources for neural regeneration in vitro [67][35], and a nerve growth factor transcript has been identified in ADSC-secreted nanovesicles that promotes synaptic growth in vitro and repair of sciatic nerve damage in vivo [88][124]. Sun et al. [89][125] proposed a new protocol called intermittent induction that alternates complete and incomplete induction media to induce ADSC differentiation into SLCs. Compared with traditional protocols, SLCs obtained by intermittent induction secrete neurotrophic factors and promote axonal growth in vitro and more effectively repair rat sciatic nerve injury in vivo. Notably, SLCs seeded in acellular nerve grafts show better functional recovery as compared with MSCs [90][126]. BMSCs [75][111], ADSCs [91][127], and UCB-MSCs [92][128] have also been seeded onto a variety of biodegradable scaffolds, with almost all resulting in better recovery relative to controls. MSCs form a neuroblast-like sheath following transplantation at the site of nerve injury and secrete neurotrophic factors that provide physical and chemical barriers for the inner nerve fibers [74][110]. Compared with polymers, acellular neural matrix hydrogels show better biocompatibility and tissue specificity and support Schwann cell proliferation in vitro and repair rat sciatic nerve defects in vivo [93][129]. Furthermore, 3D-bioprinting technology has enabled the development of 3D scaffolds with complex structures to address the challenges of nerve tissue regeneration [94][130].
For nerve regeneration studies, sciatic nerve crush and nerve gap animal models were established [95][96][131,132], and the effects of local implantation [97][133] or intravenous administration [98][99][134,135] of neural stem cells or MSCs in peripheral nerve injury models have been investigated, resulting in excellent outcomes relative to controls. ADSCs displayed relevant therapeutic potential not only via their direct release of growth factors but also through the indirect modulation of neurocyte behavior in an animal model of acute axonal injury [98][134]. Moreover, intravenously infused MSCs ameliorated function recovery post-acute peripheral nerve injury in a sciatic nerve crush model [99][135].
7. Myocardium Restoration
Cardiac disease is characterized by substantial morbidity and mortality, and serious adverse consequences. In addition to congenital heart disease, almost all cardiac diseases involve insufficient blood supply to critical regions, resulting in myocardial damage and necrosis. Although myocardium has limited regenerative capacity, restoration of severe damage to cardiomyocytes due to catastrophic myocardial infarction or other myocardial diseases is inadequate.
A role for MSC in attenuating myocardium damage was first reported in 2002 [100][136]; purified BMSCs engrafted in the murine myocardium appeared to differentiate into cardiomyocytes. Several subsequent studies evaluated the potential of different MSC sources to differentiate into cardiomyocytes [101][102][103][38,39,40], finding ADSCs as the most suitable. Spraying was found to be a more cost-effective and less invasive method for transferring ADSCs into a pig heart infarction model to promote cardiac function recovery [104][137]. MSC-specific mechanisms associated with the repair of damaged cardiomyocytes involve three factors: (1) myogenic and angiogenic capacities; (2) the ability to supply massive amounts of angiogenic, anti-apoptotic, and mitogenic factors; and (3) the inhibition of myocardial fibrosis (Figure 2) [105][106][138,139]. Butler et al. [107][140] demonstrated the safety of MSC therapy, and that it improved the left ventricular ejection fraction in patients with non-ischemic cardiomyopathy via its immunomodulatory effects. Co-culture of MSCs with cardiomyocytes promotes resistance to high oxidative stress in heart tissue after myocardial infarction [108][141]. 5-Azacytidine is an effective factor for inducing MSC differentiation into cardiomyocytes [109][142]. IGF-1-transfected MSCs protected the myocardium from fibrosis and cardiomyocyte apoptosis and reduced infarct size after myocardial infarction in rats [110][143]. Interleukin (IL)-7 enhances the fusion of MSCs with cardiomyocytes to improve cardiac function, with this attributed to the ability of IL-7 to promote cell proliferation and support damaged myocardial regeneration [111][144]. In addition to their ability to differentiate into cardiomyocytes, MSCs promoted angiogenesis by secreting vascular endothelial growth factor (VEGF) in a critical limb ischemia model [112][145], resulting in cardiac reconstruction.
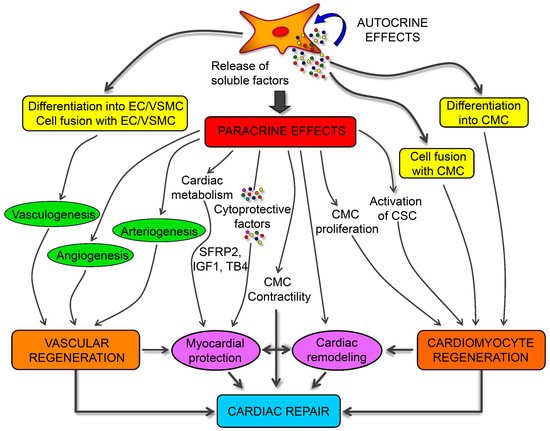
Figure 2. Schematic mechanisms of mesenchymal stem cells (MSCs) for cardiac regeneration. Angiogenesis, vasculogenesis, and cardiomyocytes differentiation capacities of MSCs make them possible for cardiac repair. Moreover, the paracrine effects of MSCs provide different kinds of growth and anti-inflammatory factors for the immunoregulation after ischemia of heart [106][139].
MSC-based therapy is a feasible strategy to improve cardiac function, according to preclinical and clinical findings [113][146]. Furthermore, MSC therapy has been intensively investigated as a treatment for myocardial infarction [114][147], peripheral ischemic vascular diseases [115][148], dilated cardiomyopathy [105][138], and pulmonary hypertension [116][149].
