Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Richard Kreider and Version 4 by Jason Zhu.
Creatine (N-(aminoiminomethyl)-N-methyl glycine) is a naturally occurring nitrogen-containing compound that plays an integral role in cellular metabolism. Creatine monohydrate (CrM) supplementation increases muscle phosphagen levels, improves repetitive high-intensity exercise performance, and promotes greater training adaptations. No significant side effects other than weight gain have been reported from CrM supplementation despite widespread use throughout the world.
- creatine monohydrate
- Bioavailability
- Stability
- Solubility
1. Introduction
While Creatine (N-(aminoiminomethyl)-N-methyl glycine) is commonly referred to as an amino acid, it is not actually an amino acid in the traditional sense. It is not incorporated into proteins or an essential, conditionally essential, and non-essential amino acid that serves as building blocks of protein. Instead, creatine is an amino acid derivative that is endogenously synthesized from the amino acids arginine and glycine by L-arginine: glycine amidinotransferase (AGAT) to guanidinoacetate (GAA). The GAA is then methylated (i.e., CH3 group added) by the enzyme guanidinoacetate N-methyltransferase (GAMT) with S-adenosyl methionine (SAMe) to form creatine [1]. The kidney, liver, pancreas, and some areas within the brain contain AGAT. Most GAA is formed in the kidney and converted by GMAT to creatine in the liver [2,3,4]. The primary role of creatine is to bind with inorganic phosphate (Pi) in the cell to form phosphocreatine (PCr), and thereby serve as a high-energy phosphate source of energy to resynthesize adenosine triphosphate (ATP) that has been degraded to adenosine diphosphate (ADP) + Pi as a source of energy to fuel cellular metabolism [5]. Creatine also plays a critical role in translocating energy-related intermediates from the electron transport system in the mitochondria to the cytosol [6,7].
About 95% of creatine is stored in the muscle, with the remaining amount found in other tissues like the heart, brain, and testes [8,9]. About two-thirds of creatine is bound with Pi and stored as PCr with the remaining one-third stored as free creatine (Cr). The total creatine pool (Cr + PCr) is about 120 mmol/kg of dry muscle mass for an individual who consumes a diet with red meat and fish [10]. The body breaks down about 1–2% of the intramuscular creatine pool into creatinine, which is excreted in the urine [10,11,12]. Daily degradation of creatine to creatinine is greater in individuals with larger muscle mass and individuals with higher levels of physical activity [13]. Creatine synthesis provides about half of the daily need for creatine [2]. The remaining creatine needed to maintain normal tissue levels is obtained in the diet primarily from red meat and fish [14,15,16,17] or dietary supplements containing a bioavailable source of creatine [14,15,16]. Since creatine stores are not fully saturated on vegan or omnivorous diets that typically provide 0 to 1.5 g/day of creatine, daily dietary creatine needs have been estimated to be 2–4 g/day [2,6,15]. For this reason, dietary supplementation of creatine has been recommended to optimize creatine stores [5,6]. The most extensively studied and effective form of creatine found in nutritional supplements that professional organizations recommend for use is creatine monohydrate (CrM) [14,15,17,18]. Over the last 30 years, several studies have shown that CrM supplementation (e.g., 5 g/day for 5–7 days or 3–5 g/day for 30 days) increases blood, muscle, and tissue levels of creatine and PCr by 20–40% [11,12,19,20]. Co-ingesting CrM with carbohydrates [20,21,22] and carbohydrate and protein [23] promotes more consistent and greater creatine retention. The increased creatine levels have been reported to enhance high-intensity exercise performance and exercise training adaptations [14,15,24]. Furthermore, there is accumulating research that CrM supplementation may have health and clinical benefits in populations that may benefit from increasing creatine availability to the cell [5].
2. Bioavailability
Bioavailability refers to the degree or rate at which a drug or substance is absorbed into the body, reaches the intended target site, and is available to influence physiological activity [1][40]. In terms of nutrients, bioavailability refers to the amount of the nutrient contained in the food or supplement that is delivered to the target tissue and available in the intended tissue for metabolic activity [1][40]. If food or supplement contains a large number of nutrients, but only a small percentage of the nutrient is liberated from the food or supplement, transported through the blood to the tissue, and ultimately taken up by the tissue, it is not very bioavailable. Similarly, if a similar quantity of food or supplement delivers less of the active nutrient to the target tissue than another food or supplement of equal quantity, it is comparatively less bioavailable. In the case of creatine, individuals who consume meat and fish in their diet typically have a plasma creatine level of around 25 µmol/L (about 3.75 µg/mL), and a muscle creatine content of about 120 mmol/kg dry muscle mass (DMM) [2][3][4][5][11,12,19,20]. Muscle creatine levels are typically lower in individuals following a vegan diet [6][7][41,42] and the elderly, who may not consume as much protein in their diet or have more difficulty digesting dietary protein [8][9][43,44]. Plasma creatine levels increase after consuming creatine-containing food and dietary supplements containing a bioavailable source of creatine in proportion to the amount creatine ingested and digestion rate [10][6][7][11][12][2,41,42,45,46].
For a dietary source of creatine to be bioavailable, creatine must be: (1) absorbed as creatine into the blood and transported to tissues [10][13][2,25]; (2) transported into tissue via tissue-specific creatine transporter genes (e.g., CRT1 or SLC6A8 in muscle) [14][15][6,7]; and (3) increase tissue and cellular creatine and PCr content by a physiologically meaningful amount to influence metabolic activity (e.g., 20–40% in muscle and 10–20% in brain) [16][3][17][5][18][19][5,12,15,20,24,47]. In creatine research, the efficacy of creatine supplementation is determined by assessing the magnitude in which creatine supplementation protocol increases muscle creatine content as typically measured from muscle biopsy samples and/or muscle and brain creatine content as determined from magnetic resonance spectroscopy (MRS) [17][15]. Since oral ingestion of creatine monohydrate (CrM) is nearly 100% bioavailable (i.e., it’s either absorbed by tissue or excreted in urine), whole-body creatine retention with CrM supplementation can also be estimated as the difference between daily intake of CrM and urinary creatine output [20][22]. Purported forms of creatine that do not increase blood creatine concentrations, do not increase uptake of creatine through tissue-specific creatine transporters, and ultimately do not increase tissue creatine levels by physiologically meaningful amounts would not affect creatine-related metabolic function. This is regardless of whether the purported form of creatine is more solubility in water, is more stable under various temperature and pH conditions outside of the body, or is delivered in food, gel, and liquid sources, as will be discussed below.
In support of this contention, classic experiments by Harris et al. [3][12] indicated that a dose of orally ingested creatine should ideally increase plasma creatine levels to greater than 500 µmol/L (75 µg/mL) to optimize tissue uptake. They reported that oral ingestion of 1 g or less of CrM had negligible effects on blood creatine content (i.e., rarely exceeding 100 µmol/L (15 µg/mL)). However, ingestion of one oral dose of 5 g of CrM (equivalent to about 1.1 kg of uncooked beef) resulted in plasma creatine levels of about 800 µmol/L (120 µg/mL) after 1 h of ingestion. It sustained plasma creatine above 200 µmol/L (30 µg/mL) for over 4–5 h. Supplementation of 5 g of CrM every 2 h maintained peak plasma creatine to levels exceeding 1000 µmol/L (150 µg/mL). Moreover, ingesting 5 g of CrM, 4 to 6 times a daily for 2-days or more significantly increased muscle creatine content of about 35%. Creatine uptake into the muscle was greatest during the first two days of CrM supplementation and declined over the next few days as muscle creatine levels became fully saturated. Subsequent studies from Hultman and colleagues [2][11] investigated the effects of various CrM supplementation strategies on changes in muscle creatine content. These experiments revealed that: (1) consuming 4 × 5 g of CrM per day for 6-days (i.e., creatine loading strategy) significantly increased muscle free creatine content by 33% and returned to baseline within 4-weeks after supplementation; (2) ingesting 4 × 5-g doses of CrM for 6-days followed by ingestion of 2 g/day of CrM for 28 days maintained a 36% increase in muscle creatine levels; and (3) ingesting 3 g/day of CrM for 35 days (i.e., low dose supplementation strategy) resulted in a gradual 16.7% increase in muscle creatine content. About 70% of the increase in the total creatine pool was observed in changes in free creatine content in the muscle.
Harris and associates [11][45] also conducted two experiments assessing the bioavailability of CrM in solution compared to creatine obtained from meat, crushed lozenges, and suspended in gel. AIn t firsthe first study, the researchers reported that ingestion of 2.5 g of CrM in solution (providing about 2.2 g of creatine) promoted a more rapid and greater increase in peak plasma creatine (287 ± 115 µmol/L or 42 µg/mL) than ingesting 408 g of lightly cooked steak containing 5.4 g (182 ± 52 µmol/L or 27 µg/mL). However, ingesting 5.4 g of creatine in lightly cooked meat promoted a more sustained increase in plasma creatine. Nevertheless, both strategies resulted in similar increases in area under the curve (AUC) values (507 ± 205 and 518 ± 153 µmol/h/L, respectively). ThenIn the second study, the researchers reported that ingestion of 2 g of CrM in solution resulted in peak plasma creatine levels of 386 ± 88 µmol/L (57 µg/mL) with an AUC of 622 ± 193 µmol/h/L. This compared to the peak value of 269 ± 67 µmol/L (41 µg/mL) with an AUC of 399 ± 196 µmol/h/L when CrM was administered in suspended gel and a peak creatine level of 277 ± 53 µmol/L and an AUC of 438 ± 131 µmol/h/L when CrM was administered as crushed lozenges. Collectively, these findings and are important because they demonstrate: (1) ingestion of 5 g of creatine from meat or 2–2.5 g of CrM administered in fluid, gels, and solids increased plasma creatine content by physiologically significant amounts needed to promote creatine uptake into the muscle; (2) the optimal single dose of CrM to increase plasma creatine levels is 5 g, but that ingesting 2–3 g will increase plasma creatine to sufficient levels to promote creatine uptake to tissue; (3) ingesting 5 g of CrM, 4 to 6 times a day for as little as 2 days was sufficient to significantly increase muscle creatine levels; (4) ingesting 5 g of CrM, 4 times a day for 6 days (i.e., 120 g total) increased muscle free creatine by about 35%; (5) consuming 3 g/day of CrM for 35 days (i.e., 105 g total) promoted a gradual 16.7% increase in muscle creatine; and (6) they provided a scientific basis for CrM supplementation recommendations and data for comparison of the efficacy of other purported forms of creatine marketed in dietary supplements [17][15].
21.1. Methods to Assess Bioavailability
21.1.1. Assess Chemical Structure
The first step in assessing the bioavailability of a purported novel form of creatine is to determine whether the purported source of creatine contains a creatine molecule. Although this seems obvious, as will be seen below, some purported sources of creatine do not contain the complete creatine molecule. Instead, they only contain a portion of the creatine molecule structure or rearrange the chemical structure such that the compound is not really creatine. Moreover, some purported sources of creatine bind or complex compounds to creatine (or a portion of its structure) with bonds so strong (e.g., amide bonds) they would not likely break down into creatine through normal digestion, and therefore would not likely increase creatine levels in the blood or tissues. In other words, they are simply not bioavailable sources of creatine. Consequently, assessing the chemical structure of a purported form of creatine and whether associated bonds would easily disassociate into a creatine molecule is the first question that should be asked when evaluating claims about a purported “novel form” of creatine.
21.1.2. Assess Changes in Blood Creatine Content
The next step is to determine if the purported source of creatine is absorbed through normal digestive processes into the blood and increases plasma creatine levels in a physiologically significant manner. Purported forms of creatine that do not significantly increase plasma creatine above normal fasting levels (i.e., about 25 µmol/L or 3.75 µg/mL) would have no effect on muscle creatine content because it would not deliver any creatine to tissues for uptake by creatine transporters. Likewise, sources of creatine that increase plasma creatine levels by less than 100 µmol/L (15 µg/mL) would not be considered viable sources of creatine for dietary supplements because they would not deliver enough creatine to target tissue to significantly increase creatine content [3][11][12,45]. Viable sources of creatine in dietary supplements should increase plasma creatine levels above 200–500 µmol/L (30–75 µg/mL) within the first hour of oral ingestion and promote a large increase in the AUC of creatine over a 4-to-5 h period [2][3][11][11,12,45]. However, an increase in plasma creatine alone does not provide definitive proof that a purported source of creatine is bioavailable or effective. Differences in plasma creatine after ingestion of a bioavailable source of creatine only suggests that absorption rates may differ. Higher blood creatine could mean that the source is not taken up as quickly into tissue, while lower levels could mean that less appears in the blood or creatine absorption into tissue is faster [21][48]. Ultimately, the source of creatine must be transported into tissues by creatine-specific transporters and increase tissue levels of creatine in physiologically significant amounts to affect creatine-related metabolism (i.e., 20–40%). Thus, it cannot be assumed that a purported source of creatine will be effectively transported into muscle based on solubility properties in fluid and/or changes in blood concentrations alone because sources of creatine that are not effectively transported into tissue could have higher plasma levels than those that promote a more rapid transport into the tissue. Consequently, it is important to assess the difference between arterial content (amount of creatine delivered to tissue) and venous creatine content or measure the amount of creatine retained in tissues directly to determine the amount of orally ingested creatine taken up by tissue.
21.1.3. Assess Changes in Tissue Creatine Content
Thus, the third step in verifying the bioavailability of a purportedly novel source of creatine is to directly assess the effects of oral ingestion at recommended dosages on tissue creatine content. This is most often done by determining changes in muscle creatine content since 95% of creatine is stored in skeletal muscle. So-called forms of creatine that have been marketed as creatine but do not have any data showing the source of creatine increases muscle creatine content in humans should not be considered a viable source of creatine until such data are available. Purported sources of creatine that increase blood levels of creatine but do not increase tissue levels of creatine in physiologically effective amounts (i.e., 20–40% in muscle and 10–20% in brain) are not bioavailable sources of creatine. Purported sources of creatine that do not deliver similar increases in muscle creatine content than equivalent doses of CrM are less bioavailable sources of creatine than CrM. Moreover, purported derivatives or analogs of creatine that have no measurable effects on plasma creatine levels and do not increase muscle creatine content are not bioavailable sources of creatine, and therefore could have no physiological effects that have been reported in the literature from CrM supplementation. Likewise, if a form of creatine does not significantly increase muscle and/or brain creatine content in humans at the recommended dosages, it should not be considered a viable source of creatine for a dietary supplement. This includes making claims that lower doses of a purportedly more bioavailable source of creatine (e.g., 1–2 g) or “sprinkling” physiologically insignificant amounts of CrM or other purported creatine sources, derivatives, or analogs of creatine in supplements or beverages (e.g., 25–250 mg) promote similar or better benefits as CrM loading (e.g., 4 × 5 g/day for 5–7 days) or long-term supplementation (e.g., 3 g/day).
32. Physio-Chemical Properties
Figure 1 shows the structure of creatine and CrM. Creatine monohydrate was the first source of creatine marketed as a dietary supplement and remains the most common source of creatine found in dietary supplements [13][25]. Creatine monohydrate is considered the gold standard to compare other purported sources of creatine because of its well-known physiochemical properties, high bioavailability, stability, low cost, and a large number of studies that have demonstrated efficacy and safety [17][13][15,25]. CrM has been so extensively studied compared to other purported forms of creatine that when creatine supplementation is discussed in the literature, it is understood the researcheuthors are referring to CrM unless otherwise specified [17][22][23][24][15,17,18,49]. Nevertheless, CrM is formed by crystallization with water forming monoclinic prisms that hold one molecule of water per molecule of creatine [13][25]. This provides a powder containing 87.9% creatine that readily dissociates into creatine and water upon oral ingestion. The water in CrM can also be removed when exposed to heat at about 100 °C yielding anhydrous creatine that is 100% creatine [13][25]. However, due to the increased temperature used during the drying, anhydrous creatine contains higher amounts of the impurity creatinine. Creatine appears as internal salt and is considered a fairly weak base (pKb 11.02 at 25 °C) that forms salts with strong acids (i.e., pKa < 3.98) [13][25]. Creatine can also complex with other compounds via ionic binding (i.e., the attraction of positive cation and negative anion charges).
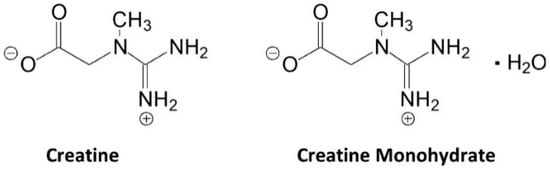
Figure 1. Chemical structure of creatine and creatine monohydrate.
Pischel and Gastner [25][50] described the basic process of industrial synthesis of CrM. The process involves adding acetic acid to an aqueous sodium sarconsinate solution and stirring to a pH value of about 10 and a temperature of about 80 °C. An aqueous cyanamide solution is then added to the medium and agitated to facilitate the reaction. After cooling, the crystalline CrM is filtered, separated, and then dried [25][50]. Creatine monohydrate is manufactured by using water as solvent in Germany has produced 99.9% pure CrM with no contaminants under the brand name Creapure®. Other sources of CrM, particularly from China, have been reported to contain contaminates like dicyandiamide, dihydrotriazine, dimethyl sulphate, thiourea, creatinine, and/or higher concentrations of heavy metals like mercury and lead due to the use of different chemical precursors, poorly controlled synthesis processes, using organic solvents, and/or less than adequate filtration methods that increase production of these contaminants [25][50]. For this reason, creatine monohydrate manufactured by AlzChem in Germany is considered the gold standard of creatine and has been the primary source of creatine used in hundreds of clinical trials conducted on CrM to establish safety and efficacy [17][13][15,25].
43. Stability
Creatine monohydrate is very stable in powder form, showing no signs of degradation to creatinine over years, even at elevated temperatures [13][25]. For example, Jäger [26][51] reported that CrM powder showed no signs of degrading to creatinine even with temperatures up to 40 °C (104 °F) for more than three years. Additionally, even when CrM was stored at 60 °C (140 °F), creatinine could only be detected in trace amounts after 44 months of storage [26][51]. However, creatine is not as stable in solution due to intramolecular cyclization that converts creatine to creatinine. Generally, creatine degrades to creatinine in solution at a faster rate as pH decreases and temperature increases [13][27][28][29][25,52,53,54]. For example, Harris and coworkers [30][55] reported that creatine is relatively stable for 3 days in solution at neutral pH (6.5 to 7.5) However, the rate of degradation to creatinine increased when stored at 25 °C when pH decreased (e.g., 4% at 5.5 pH; 12% at 4.5 pH; and 21% at 3.5 pH) [30][55]. However, as described below, the conversion of creatine to creatinine is halted at pH levels < 2.5. For this reason, it is recommended that CrM should be consumed immediately after it is mixed in an acidic beverage or refrigerated to slow the degradation to creatinine and consumed within a couple of days. However, recent reports presented shelf-life stability data of CrM suspended in a solution of 70% for 13-months at neutral pH and 100% for 12 months at a pH of 2.8 [31][32][56,57].
As mentioned above, the degradation of creatine can be limited or prevented when creatine is in very low or very high pH environments [13][25]. In this regard, a pH > 12.1 promotes the deprotonation of the acid group. This makes it more difficult for intramolecular cyclization to form creatinine [13][25]. On the other hand, when pH is <2.5, the amide functional group on the creatine molecule is protonated and prevents the intramolecular cyclization[13][25]. Since stomach acid is generally less than 2.5, less than 1% of CrM is degraded to creatinine during digestion and 99% of creatine is taken up by tissue or excreted in urine after ingestion [3][13][33][34][12,25,58,59].
54. Solubility
One of the limitations in terms of developing consumer products containing CrM is that CrM powder is not highly soluble. For example, when mixing CrM in solution, some CrM residue remains at the bottom of the glass requiring consumers to add more fluid, swirl, and quickly ingest to ensure they consumed all the creatine. While this has no effect on creatine bioavailability as CrM is nearly 100% bioavailable [3][13][33][34][12,25,58,59], there has been interest in finding ways to improve the solubility of creatine. The solubility of creatine in water increases linearly with increasing temperature. In this regard, about 6 g of creatine dissolves in one liter of water at 4 °C (39.2 °F) while 14 g/L are dissolved at 20 °C (68 °F), 34 g/L are dissolved at 50 °C (122 °F); and, 45 g/L are dissolved at 60 °C (140 °F) [13][25]. This is the reason that some researchers initially administered CrM to participants in warm to hot water [3][12] or hot tea [35][60]. Creatine solubility can also be improved by administering CrM in lower pH solutions like juices and sport drinks that generally have pH levels ranging from 2.5–3.5 [36][61] and/or blending CrM with carbohydrate and/or protein powders or in juice which helps suspend CrM in solution, reduce sedimentation, and enhance creatine uptake into muscle [5][37][20][38][39][20,21,22,23,62].
Dissolving creatine in more acidic environment is also the rationale in providing creatine in the form of easily disassociated creatine salts. Adding an acidic moiety to a creatine molecule lowers the pH of the water. For example, adding tri-creatine citrate to water yields a pH of 3.2 and increases solubility to 29 g/L whereas adding creatine pyruvate to water yields a pH of 2.6 and increases solubility to 54 g/L [40][63]. Creatine hydrochloride (HCl) has also been marketed as a pH lowering source of creatine with greater solubility than CrM [41][42][64,65]. While lowering pH and/or mixing creatine salts into solution enhances solubility, the amount of creatine contained in these forms of creatine salt must be equalized to CrM to deliver the same amount of creatine to the blood and tissues. In this regard, CrM contains 87.9% creatine whereas creatine citrate (40.6%), di-creatine citrate (57.7%), creatine pyruvate (59.8%), and creatine HCl (78.2%) contain less creatine by weight compared to CrM. Therefore, one would need to mix 1.54, 1.34, 1.32, and 1.11 times more of these forms of creatine in solution to deliver the same amount of creatine than CrM. Additionally, while mixing CrM in common juices and beverages with pHs ranging from 2.5–3.5 [36][61] would enhance solubility, it would also promote the conversion of creatine to creatinine over time [43][66]. Therefore, it is best to consume creatine salts or CrM with acid beverages soon after it is mixed so that the conversion of creatine to creatinine would be halted upon entering a more acidic environment in the stomach.
With that said, the only real advantage of mixing CrM in an acidic beverage is that it would leave less CrM in crystalized form at the bottom of a cup to swirl and consume during the last drink. If an individual consumes all the CrM (soluble or not), it will be bioavailability in terms of intestinal absorption, transport of creatine in the blood, transport of creatine through tissue-specific creatine transporters, and uptake of creatine into tissues. Similarly, if a bioavailable source of creatine is consumed at physiologically effective doses, it is not degraded during digestion, and it increases blood and tissue creatine content by physiologically meaningful amounts (i.e., 20–40%), it does not matter whether a form of creatine has better mixing characteristics and/or is more soluble. Research has clearly shown that CrM is not degraded into creatinine during normal digestion, it is nearly 100% bioavailable [3][13][33][34][12,25,58,59], and markedly increases blood and tissue creatine content. There are no data showing that any other purported form of creatine is more effective in increasing tissue creatine content than CrM [39][62]. Therefore, claims that a given form of creatine is more effectively absorbed than CrM because it is more soluble in water is unsupported marketing hyperbole.
