Chronic use of glyceryl trinitrate (GTN) is limited by serious side effects, such as tolerance and endothelial dysfunction of coronary and resistance arteries. Although GTN is used as a drug since more than 130 years, the mechanisms of the vasodilatory effects and of tolerance development to organic nitrates are still incompletely elucidated. New synthesized organic nitrates with and without antioxidant properties were characterized for their ex vivo tolerance profile, in order to investigate the oxidative stress hypothesis of nitrate tolerance. The organic nitrates studied showed different vasodilation and tolerance profiles, probably due to the ability or inability of the compounds to interact with the aldehyde dehydrogenase-2 enzyme (ALDH-2) involved in bioactivation. Furthermore, nitrooxy derivatives endowed with antioxidant properties did not determine the onset of tolerance, even if bioactivated by ALDH-2. The results could be further evidence of the involvement of ALDH-2 in the development of nitrate tolerance. Moreover, the behavior of organic nitrates with antioxidant properties supports the hypothesis of the involvement of ROS in inactivating ALDH-2.
- multitarget drugs
- tolerance
- antioxidants
- organic nitrates
- aldehyde dehydrogenase 2
1. Introduction
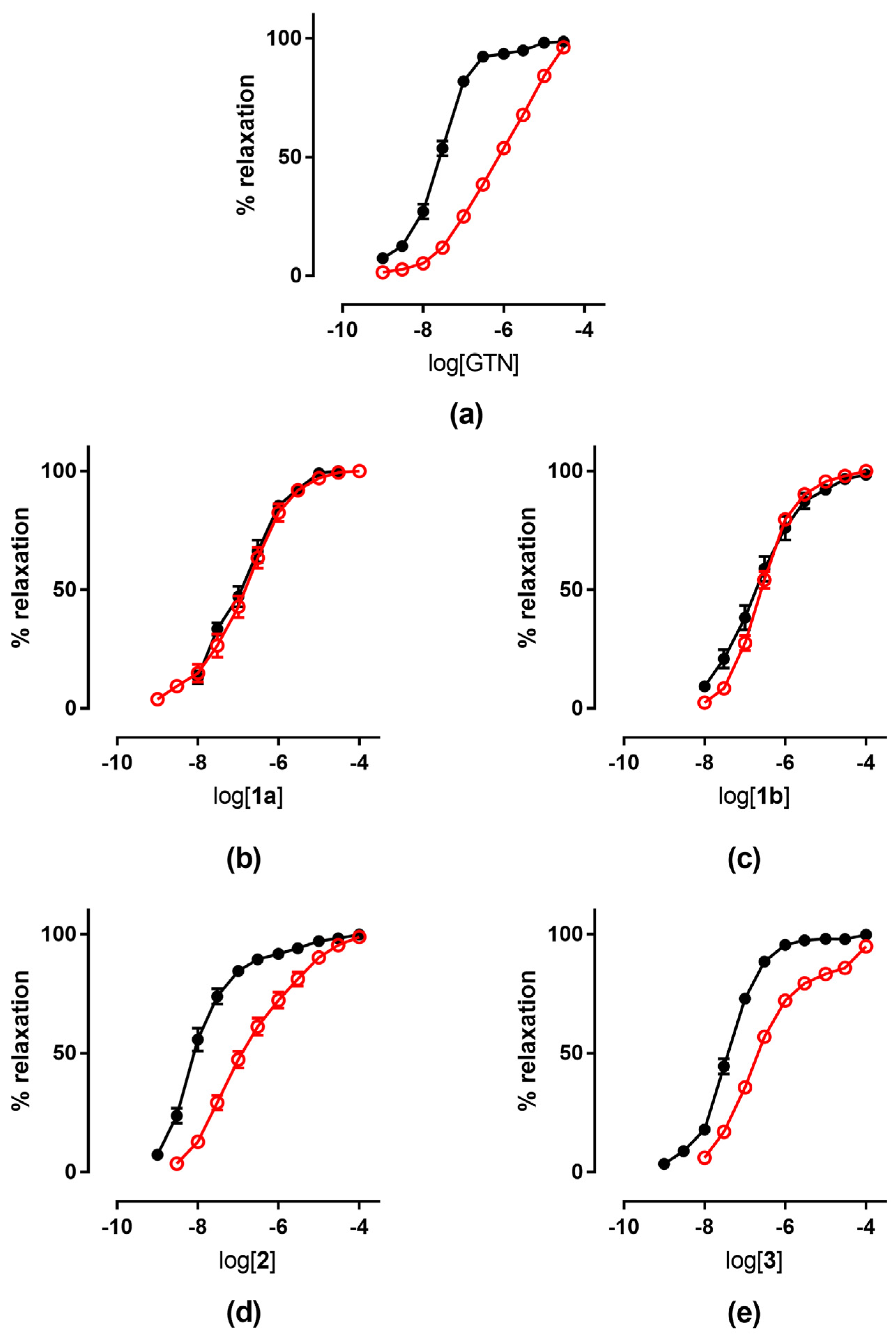
2. Vasodilating Activity
2.1. In Vitro Experiments
| In Vitro Experiments pEC50 ± SE |
Ex Vivo Experiments pEC50 ± SE |
||||
|---|---|---|---|---|---|
| Compd | + Benomyl 1 | + Chloral Hydrate 2 |
Control | Tolerant Vessels |
|
| 1a | 6.68 ± 0.08 3 | 6.49 ± 0.10 3 | 6.92 ± 0.06 | 6.80 ± 0.05 | |
| 1b | 6.66 ± 0.07 3 | 6.46 ± 0.10 3 | 6.57 ± 0.03 3 | 6.70 ± 0.12 | 6.49 ± 0.06 |
| 2 | 7.20 ± 0.15 3 | 6.20 ± 0.12 3 | 5.92 ± 0.3 3 | 7.71 ± 0.11 | 6.67 ± 0.09 4 |
| 3 | 6.80 ± 0.07 3 | 5.82 ± 0.5 3 | 5.52 ± 0.5 3 | 7.36 ± 0.04 | 6.40 ± 0.06 5 |
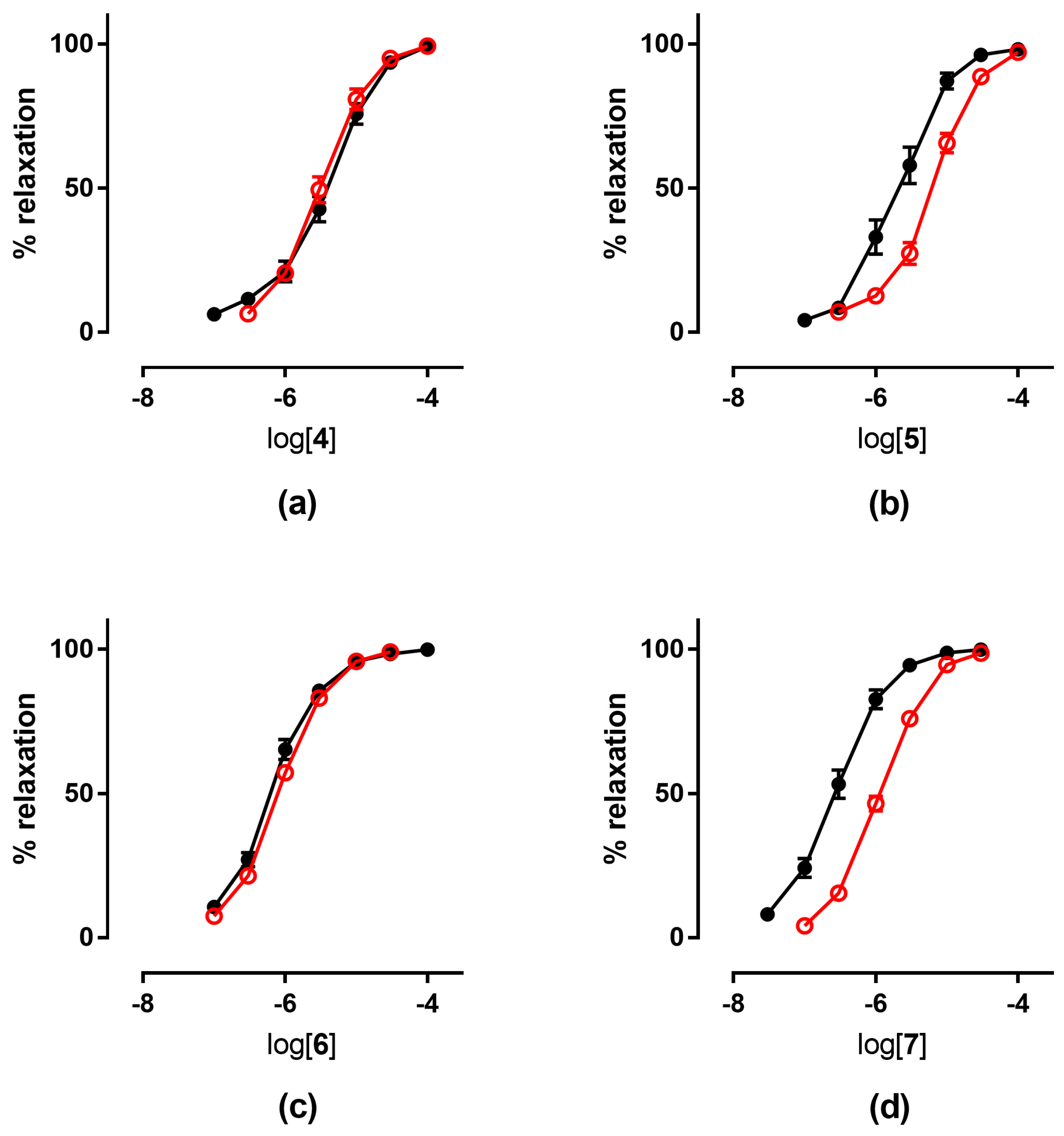
| 4 | 5.48 ± 0.09 | 4.85 ± 0.07 | 4.79 ± 0.11 | 5.48 ± 0.08 | 5.49 ± 0.05 |
| 5 | 5.52 ± 0.09 | 4.72 ± 0.07 | 4.68 ± 0.03 | 5.64 ± 0.08 | 5.22 ± 0.05 6 |
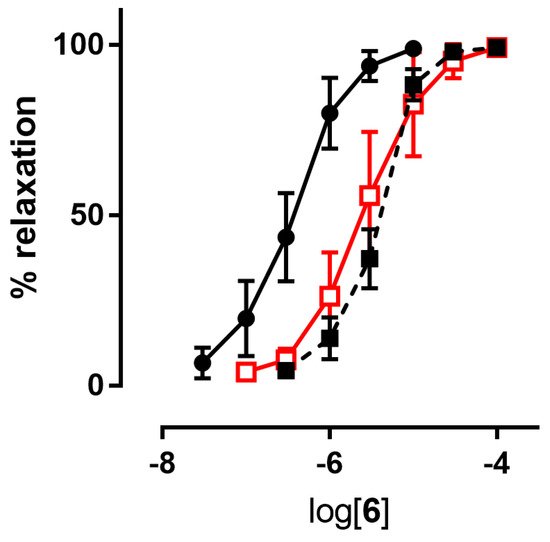
| 6 | 6.19 ± 0.09 | 5.48 ± 0.08 | 5.59 ± 0.16 | 6.16 ± 0.04 | 6.06 ± 0.03 |
| 7 | 6.62 ± 0.10 | 5.58 ± 0.08 | 5.52 ± 0.06 | 6.55 ± 0.07 | 5.88 ± 0.02 7 |
| GTN | 7.54 ± 0.043 | 6.38 ± 0.07 3 | 6.03 ± 0.05 3 | 7.52 ± 0.04 | 6.10 ± 0.08 |
2.2. Ex Vivo Experiments
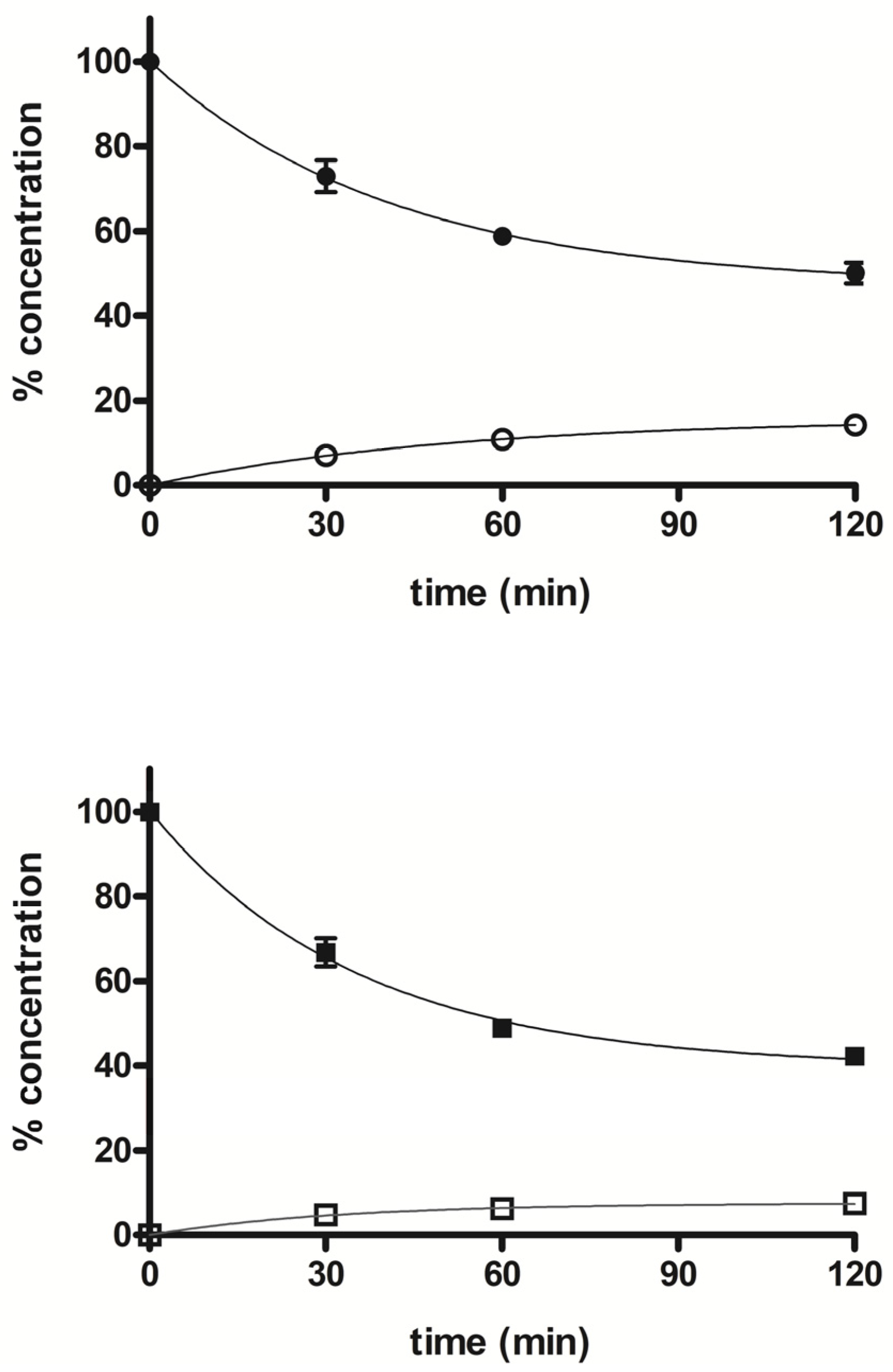
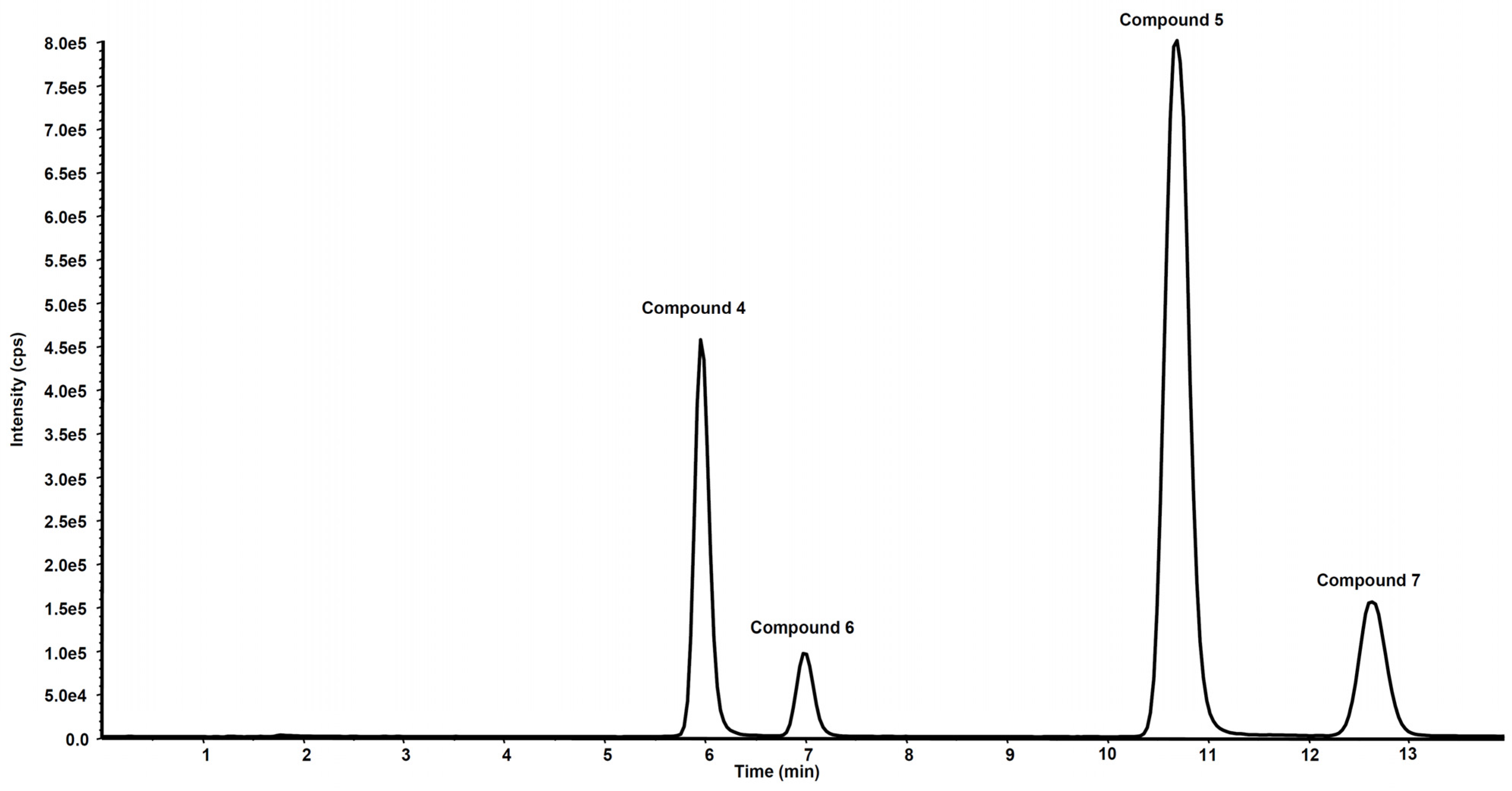
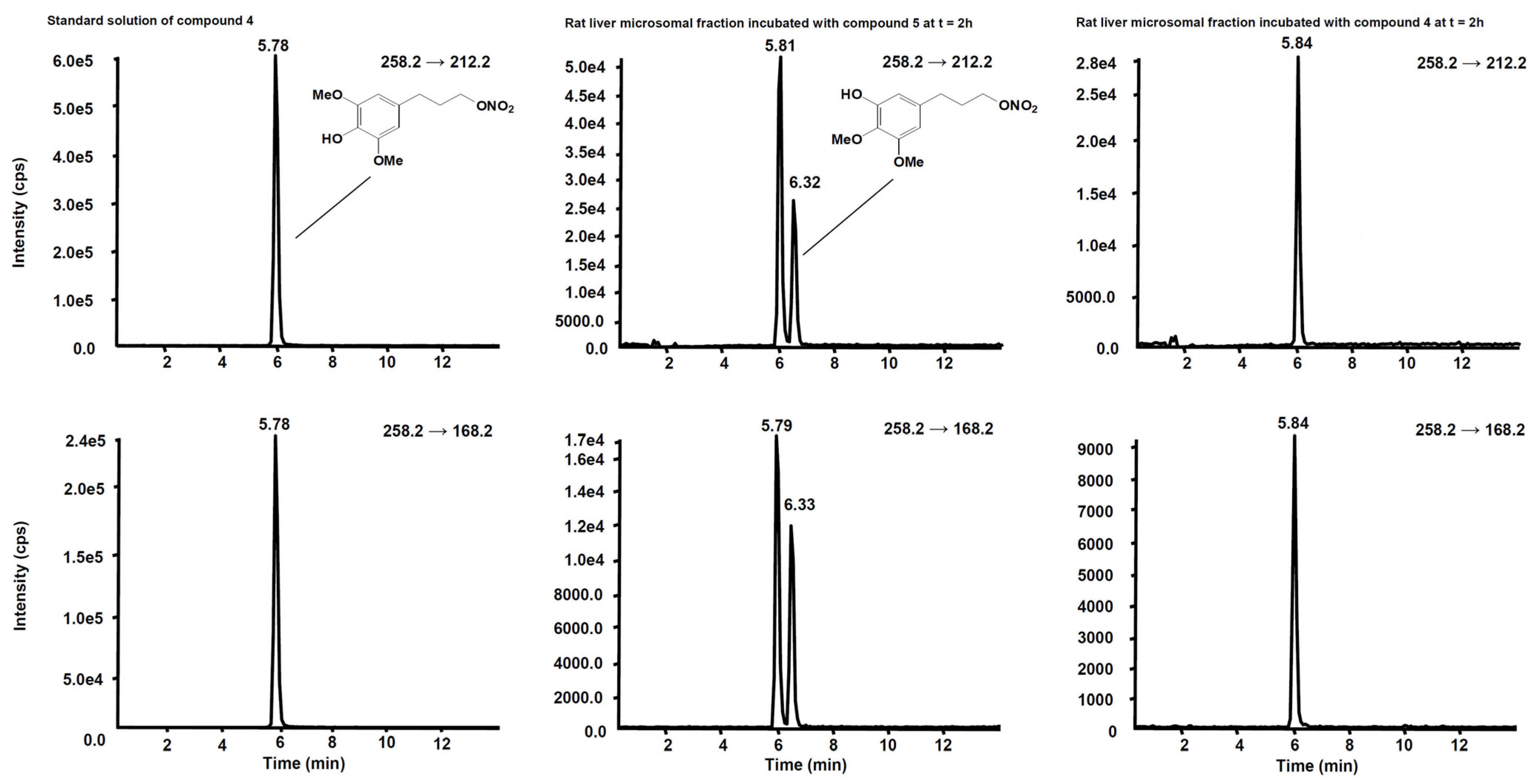
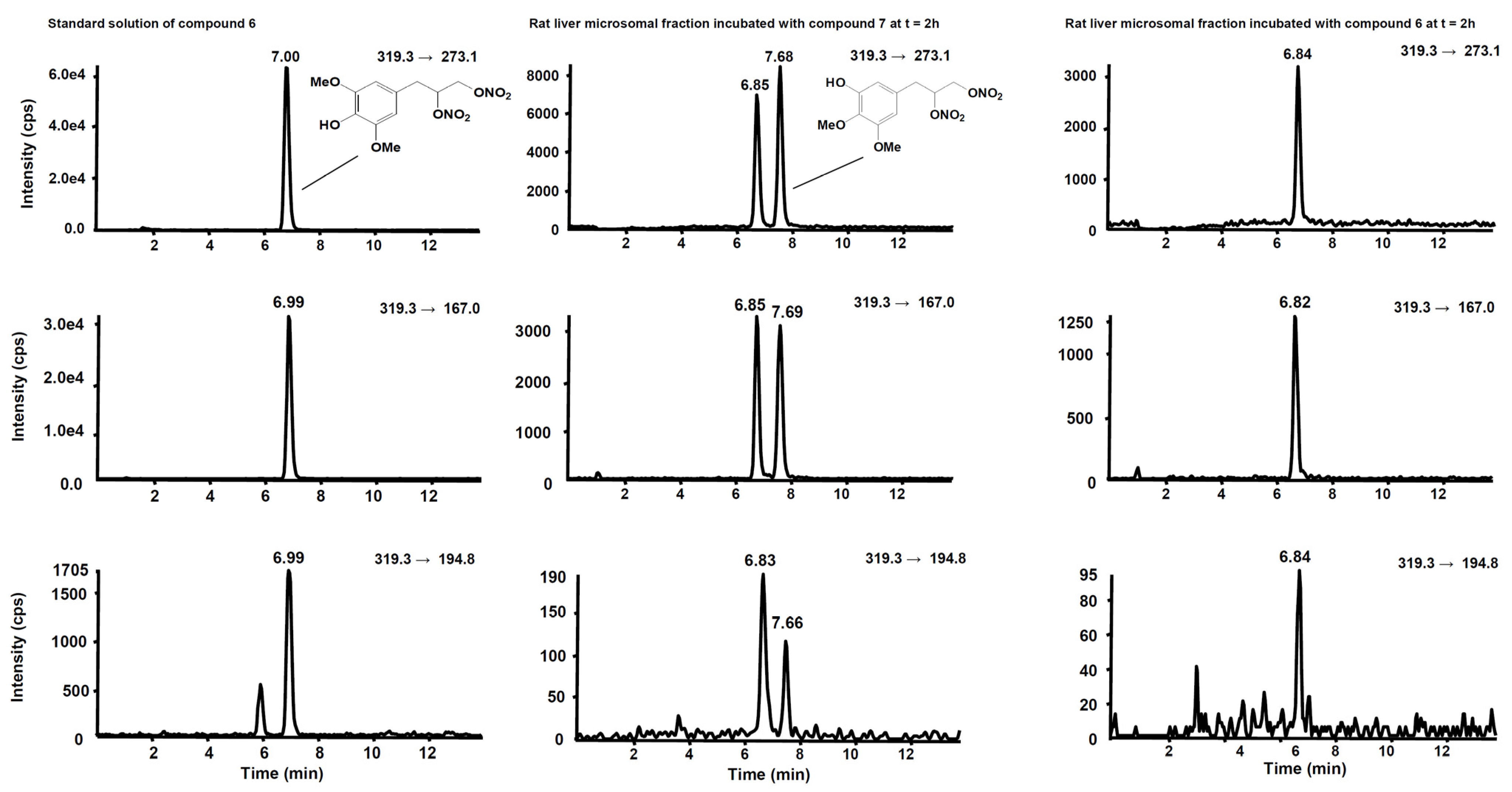
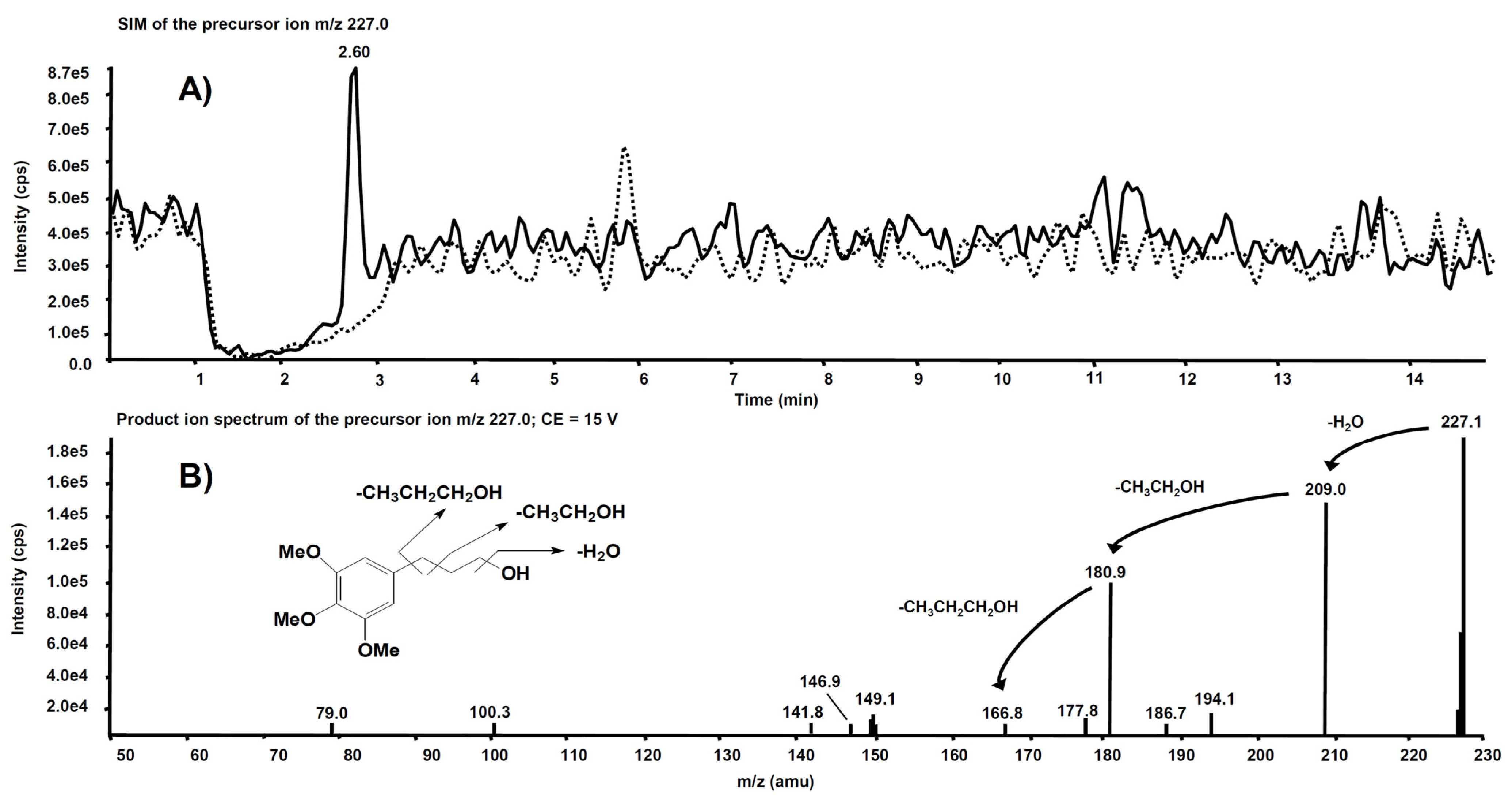
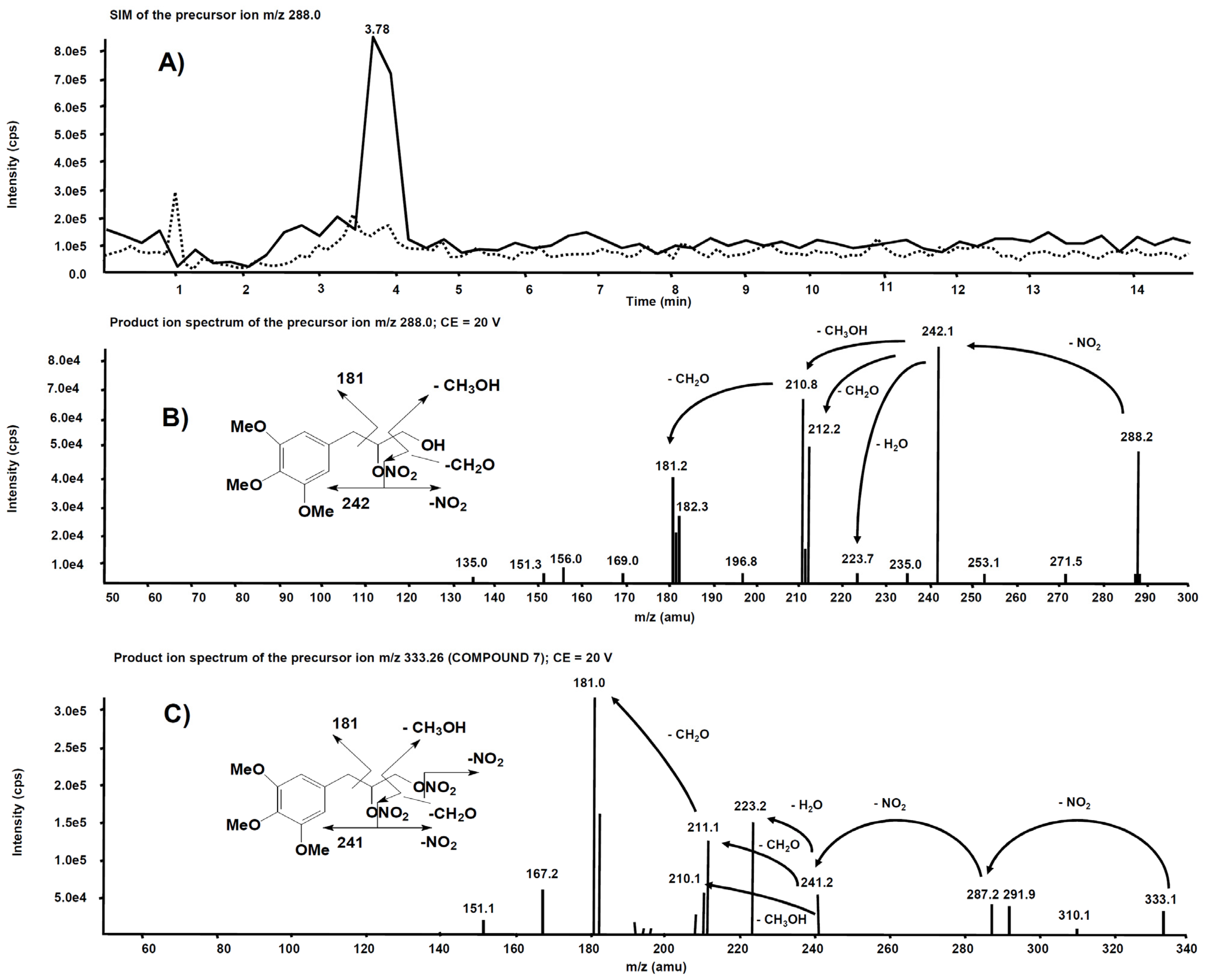
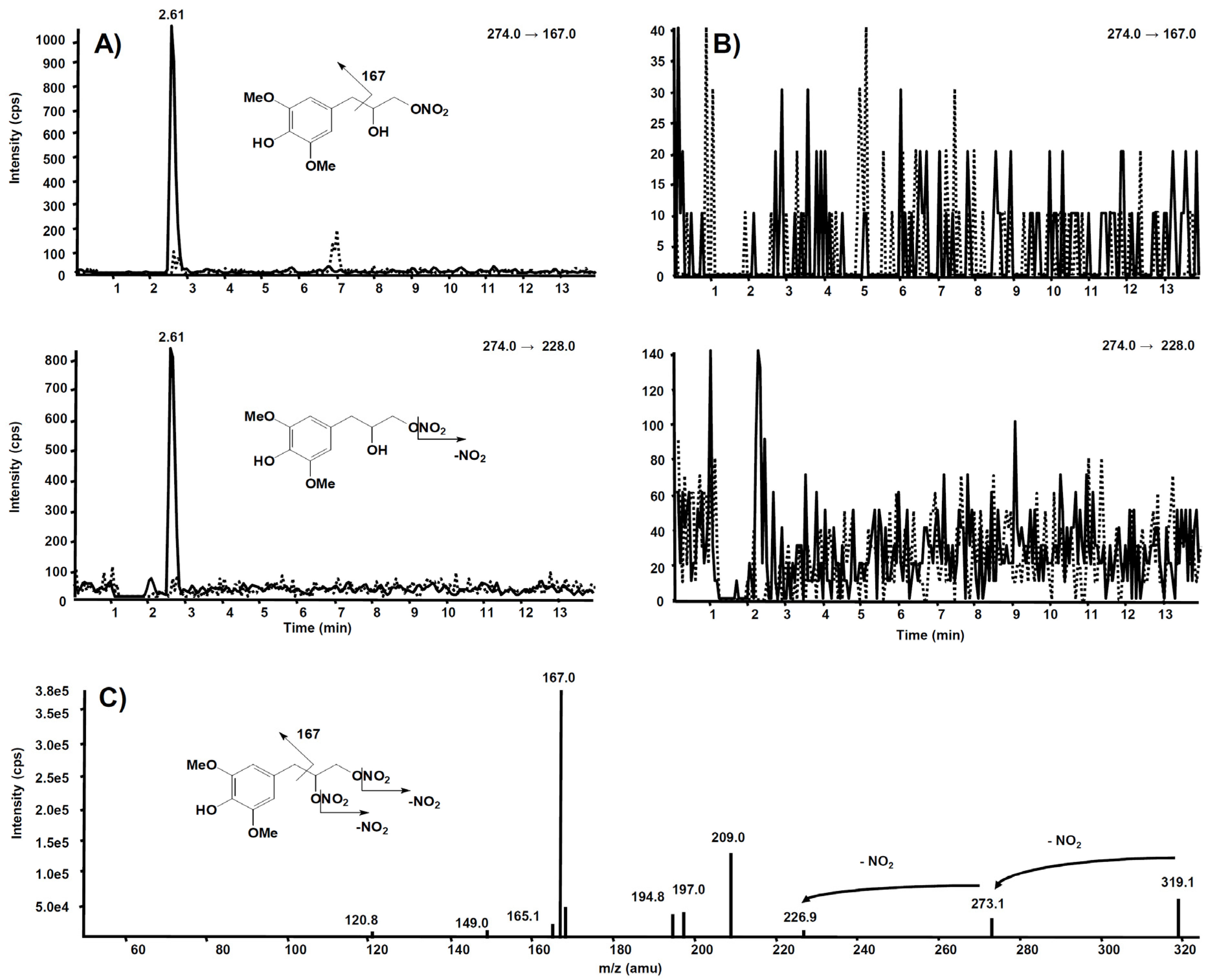
3. Metabolism
| Compd | Precurson Ion (m/z) | Declustering Potential (V) | Entrance Potential (V) | Product Ions | Collision Energy (V) | Collision Cell Exit Potential (V) |
|---|---|---|---|---|---|---|
| 4 | 258.2 | 30 | 4 | 258.2 → 212.2 | 12 | 15 |
| 258.2 → 168.2 | 22 | 15 | ||||
| 5 | 272.1 | 29 | 8 | 272.1 → 226.2 | 13 | 18 |
| 272.1 → 182.2 | 20 | 14 | ||||
| 272.1 → 211.1 | 22 | 20 | ||||
| 6 | 319.3 | 63 | 9 | 319.3 → 273.1 | 10 | 18 |
| 319.3 → 167.0 | 18 | 16 | ||||
| 319.3 → 194.8 | 19 | 25 | ||||
| 7 | 333.2 | 40 | 9 | 333.2 → 181.0 | 18 | 15 |
| 333.2 → 167.1 | 34 | 30 | ||||
| 333.2 → 223.2 | 17 | 17 |
References
- Fung, H.L. Biochemical mechanism of nitroglycerin action and tolerance: Is this old mistery solved? Annu. Rev. Pharmacol. Toxicol. 2004, 44, 67–85.
- Daiber, A.; Münzel, T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: Emphasis on redox biology and oxidative stress. Antioxid. Redox Signal. 2015, 23, 899–946.
- Lopez, M.; Malacarne, P.F.; Gajos-Draus, A.; Ding, X.; Daiber, A.; Lundberg, J.O.; Offermanns, S.; Brandes, R.P.; Rezende, F. Vacular biotransformation of organic nitrates is independent of cytochrome P450 monooxygenases. Br. J. Pharmacol. 2021, 178, 1495–1506.
- Chen, Z.; Zhang, J.; Stamler, J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 8306–8311.
- Tsikas, D.; Surdacki, A. Biotransformation of organic nitrates by glutathione S-transferases and other enzymes: An appraisal of the pioneering work by William B. Jakoby. Anal. Biochem. 2020, 113993.
- Opelt, M.; Eroglu, E.; Waldeck-Weiermair, M.; Russwurm, M.; Koesling, D.; Malli, R.; Graier, W.F.; Fassett, J.T.; Schrammel, A.; Mayer, B. Formation of nitric oxide by aldehyde dehydrogenase-2 is necessary and sufficient for vascular bioactivation of nitroglycerin. J. Biol. Chem. 2016, 291, 24076–24084.
- Dudek, M.; Bednarski, M.; Bilska, A.; Iciek, M.; Sokolowska-Jezewicz, M.; Filipek, B.; Wlodek, L. The role of lipoic acid in prevention of nitroglicerin tolerance. Eur. J. Pharmacol. 2008, 591, 203–210.
- Gori, T. Exogenous NO therapy for the treatment and prevention of atherosclerosis. Int. J. Mol. Sci. 2020, 21, 2703.
- Daiber, A.; Oelze, M.; Wenzel, P.; Bollmann, F.; Pautz, A.; Kleinert, H. Heme oxygenase-1 induction and organic nitrate therapy: Beneficial effects on endothelial dysfunction, nitrate tolerance, and vascular oxidative stress. Int. J. Hypertens. 2012, 2012, 842632.
- Mizuno, Y.; Harada, E.; Kugimiya, F.; Shono, M.; Kusumegi, I.; Yoshimura, M.; Kinoshita, K.; Yasue, H. East Asian variant mitochondrial aldehyde dehydrogenase-2 genotype exacerbates nitrate tolerance in patients with coronary spastic angina. Circ. J. 2020, 84, 479–486.
- Münzel, T.; Daiber, A.; Mülsch, A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005, 97, 618–628.
- Omidkhoda, S.F.; Razavi, B.M.; Imenshahidi, M.; Rameshrad, M.; Hosseinzadeh, H. Evaluation of possible effects of crocin against nitrate tolerance and endothelial dysfunction. Iran. J. Basic Med. Sci. 2020, 23, 303–310.
- Münzel, T.; Sayegh, H.; Freeman, B.A.; Tarpey, M.M.; Harrison, D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Investig. 1995, 95, 187–194.
- Khong, S.M.L.; Andrews, K.L.; Huynh, N.N.; Venardos, K.; Aprico, A.; Michell, D.L.; Zarei, M.; Moe, K.T.; Dusting, G.J.; Kaye, D.M.; et al. Arginase II inhibition prevents nitrate tolerance. Br. J. Pharmacol. 2012, 166, 2015–2023.
- Sage, P.R.; de la Lande, I.S.; Stafford, I.; Bennett, C.L.; Phillipov, G.; Stubberfield, J.; Horowitz, J.D. Nitroglycerin tolerance in human vessels: Evidence of impaired nitroglycerin bioconversion. Circulation 2000, 102, 2810–2815.
- Gongadze, N.; Kezeli, T.D.; Sukoyan, G.V.; Chapichadze, Z.; Dolidze, N.M.; Mirziashvili, M.; Chipashvili, M. Deterioration in hemodynamics reaction, baroreflex sensitivity, sympathetic nerve activity and redox state of thoracic aorta in the experimental model of nitrate tolerance and its pharmacological correction. Pharmacol. Pharm. 2016, 7, 81–88.
- Mayer, B.; Beretta, M. The enigma of nitroglycerin bioactivation and nitrate tolerance: News, views and troubles. Br. J. Pharmacol. 2008, 155, 170–184.
- Daiber, A.; Wenzel, P.; Oelze, M.; Münzel, T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin. Res. Cardiol. 2008, 97, 12–20.
- Münzel, T.; Steven, S.; Daiber, A. Organic nitrates: Update on mechanism underlying vasodilation, tolerance and endothelial dysfunction. Vasc. Pharmacol. 2014, 63, 105–113.
- Esplugues, J.V.; Rocha, M.; Nuñez, C.; Bosca, I.; Ibiza, S.; Herance, J.R.; Ortega, A.; Serrador, J.M.; D’Ocon, P.; Victor, V.M. Complex I dysfunction and tolerance to nitroglycerin—An approach based on mithocondrial-targeted antioxidants. Circ. Res. 2006, 99, 1067–1075.
- Opelt, M.; Wölkart, G.; Eroglu, E.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F.; Kollau, A.; Fassett, J.T.; Schrammel, A.; Mayer, B.; et al. Sustained formation of nitroglycerin-derived nitric oxide by aldehyde dehydrogenase-2 in vascular smooth muscle without added reductants: Implications for the development of nitrate tolerance. Mol. Pharmacol. 2018, 93, 335–343.
- Chegaev, K.; Lazzarato, L.; Marcarino, P.; Di Stilo, A.; Fruttero, R.; Vanthuyne, N.; Roussel, C.; Gasco, A. Synthesis of some novel organic nitrates and comparative in vitro study of their vasodilator profile. J. Med. Chem. 2009, 52, 4020–4025.
- Boschi, D.; Tron, G.C.; Lazzarato, L.; Chegaev, K.; Cena, C.; Di Stilo, A.; Giorgis, M.; Bertinaria, M.; Fruttero, R.; Gasco, A. NO-Donor phenols: A new class of products endowed with antioxidant and vasodilator properties. J. Med. Chem. 2006, 49, 2886–2897.
- Tosco, P.; Marini, E.; Rolando, B.; Lazzarato, L.; Cena, C.; Bertinaria, M.; Fruttero, R.; Reist, M.; Carrupt, P.A.; Gasco, A. Structure-antioxidant activity relationships in a series of NO-donor phenols. ChemMedChem 2008, 3, 1443–1448.
- Limbu, R.; Cottrell, G.S.; McNeish, A.J. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS ONE 2018, 13, e0192484.
- Shimokawa, H.; Godo, S. Diverse Functions of Endothelial NO Synthases System: NO and EDH. J. Cardiovasc. Pharmacol. 2016, 67, 361–366.
- Fontaine, D.; Otto, A.; Fontaine, J.; Berkenboom, G. Prevention of nitrate tolerance by long-term treatment with statins. Cardiovasc. Drugs Ther. 2003, 17, 123–128.
