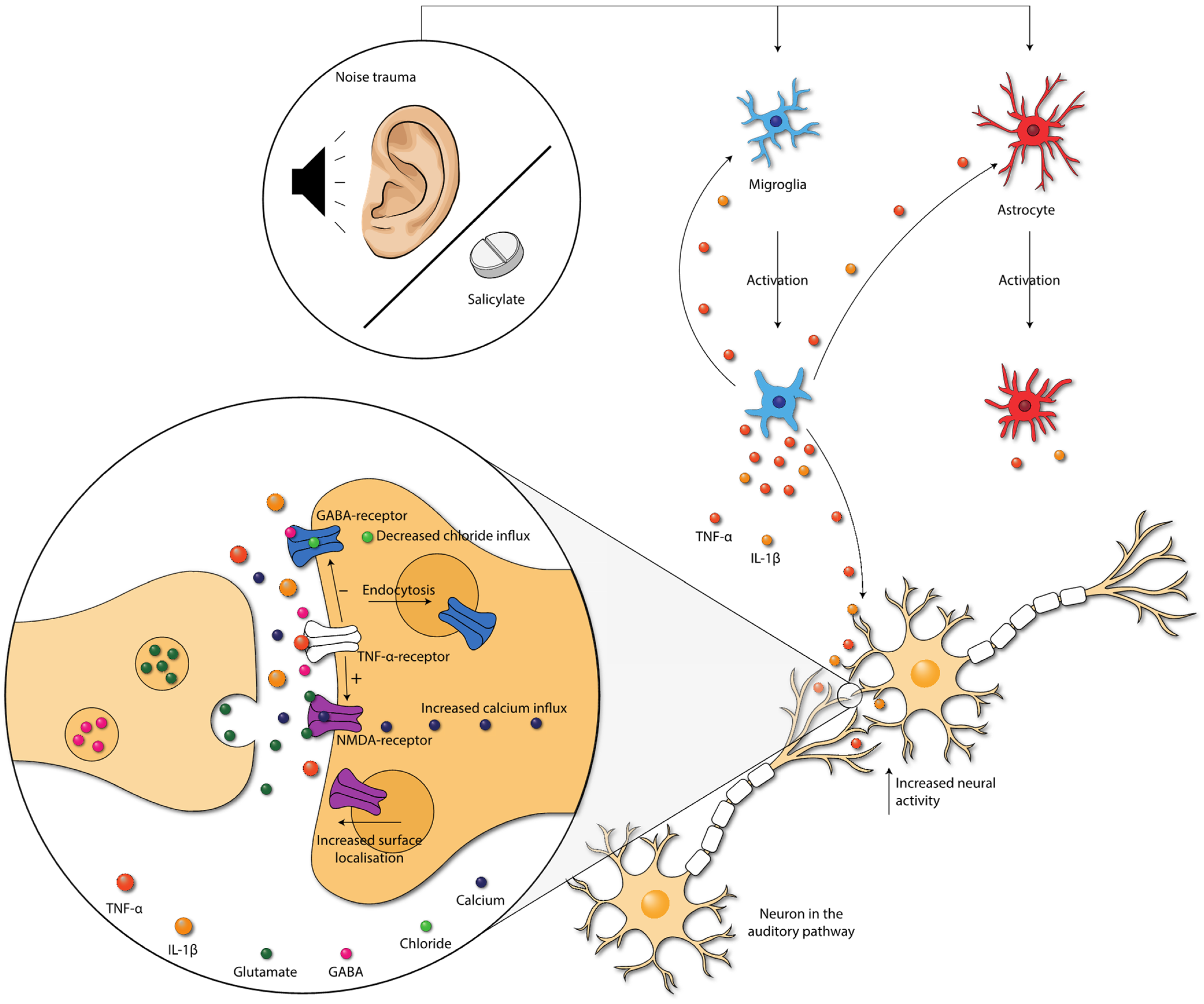
Accumulating evidence suggests that inflammation plays a role in the pathogenesis of subjective tinnitus. Noise exposure and salicylate administration both lead to inflammation throughout the whole auditory pathway. In particular, TNF-α, IL-1β, glia and activated platelets are associated with acute tinnitus. TNF-α and IL-1β influence NMDA and GABA receptors, leading to an increased excitatory and decreased inhibitory neurotransmission. These changes can lead to neuroplasticity and thus chronic tinnitus. Whether inflammatory mediators still play a role in chronic tinnitus remains to be elucidated. Nevertheless, drugs targeting the involved inflammatory mediators could be a potential effective treatment for (acute) tinnitus.
- tinnitus
- inflammation
- microglia
- astrocytes
- cytokines
- platelets
1. Introduction
2. Current Insights
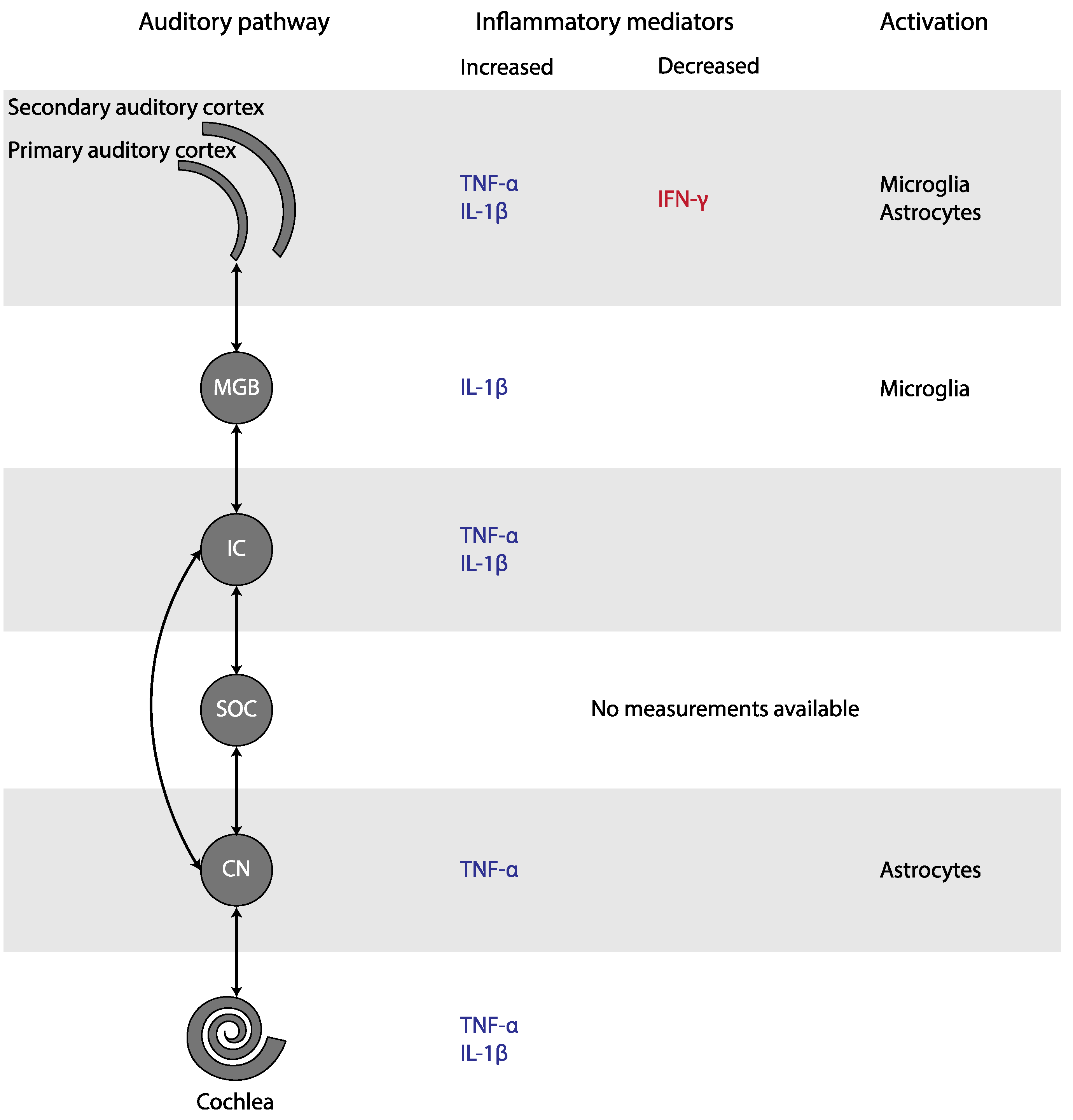
2.1. Cytokine Involvement in Tinnitus
2.1.1. TNF-α in Tinnitus
2.1.2. IL-1β in Tinnitus
2.2. Neuroglial Involvement in Tinnitus
2.3. Platelet Involvement in Tinnitus
2.4. Inflammation in the Pathophysiology of Tinnitus
2.5. Challenges in the Interpretation of Results on Inflammation in Tinnitus
2.5.1. Translation from Animal to Human
2.5.2. Contribution of Hearing Loss
2.6. Potential Treatment Options
2.6.1. Treatments Targeting Cytokines
2.6.2. Treatments Targeting Microglia
References
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A Systematic Review of the Reporting of Tinnitus Prevalence and Severity. Hear. Res. 2016, 337, 70–79.
- Eggermont, J.J.; Roberts, L.E. The Neuroscience of Tinnitus. Trends Neurosci. 2004, 27, 676–682.
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607.
- Knipper, M.; Van Dijk, P.; Nunes, I.; Rüttiger, L.; Zimmermann, U. Advances in the Neurobiology of Hearing Disorders: Recent Developments Regarding the Basis of Tinnitus and Hyperacusis. Prog. Neurobiol. 2013, 111, 17–33.
- Fuentes-Santamaría, V.; Alvarado, J.C.; Melgar-Rojas, P.; Gabaldón-Ull, M.C.; Miller, J.M.; Juiz, J.M. The Role of Glia in the Peripheral and Central Auditory System Following Noise Overexposure: Contribution of TNF-α and IL-1β to the Pathogenesis of Hearing Loss. Front. Neuroanat. 2017, 11, 9.
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation Associated with Noise-Induced Hearing Loss. J. Acoust. Soc. Am. 2019, 146, 4020–4032.
- Pan, W.; Zadina, J.E.; Harlan, R.E.; Weber, J.T.; Banks, W.A.; Kastin, A.J. Tumor Necrosis Factor-α: A Neuromodulator in the CNS. Neurosci. Biobehav. Rev. 1997, 21, 603–613.
- Wang, W.; Zhang, L.S.; Zinsmaier, A.K.; Patterson, G.; Leptich, E.J.; Shoemaker, S.L.; Yatskievych, T.A.; Gibboni, R.; Pace, E.; Luo, H.; et al. Neuroinflammation Mediates Noise-Induced Synaptic Imbalance and Tinnitus in Rodent Models. PLoS Biol. 2019, 17, e3000307.
- Hu, S.S.; Mei, L.; Chen, J.Y.; Huang, Z.W.; Wu, H. Effects of Salicylate on the Inflammatory Genes Expression and Synaptic Ultrastructure in the Cochlear Nucleus of Rats. Inflammation 2014, 37, 365–373.
- Chen, X.H.; Zheng, L.L. Expression of Pro-Inflammatory Cytokines in the Auditory Cortex of Rats with Salicylate-Induced Tinnitus. Mol. Med. Rep. 2017, 16, 5643–5648.
- Hwang, J.-H.; Chen, J.-C.; Chan, Y.-C. Effects of C-Phycocyanin and Spirulina on Salicylate-Induced Tinnitus, Expression of NMDA Receptor and Inflammatory Genes. PLoS ONE 2013, 8, e58215.
- Jang, C.H.; Lee, S.; Park, I.Y.; Song, A.; Moon, C.; Cho, G.-W. Memantine Attenuates Salicylate-Induced Tinnitus Possibly by Reducing NR2B Expression in Auditory Cortex of Rat. Exp. Neurobiol. 2019, 28, 495–503.
- Nguyen, M.D.; Julien, J.P.; Rivest, S. Innate Immunity: The Missing Link in Neuroprotection and Neurodegeneration? Nat. Rev. Neurosci. 2002, 3, 216–227.
- Haider, H.F.; Ribeiro, S.F.; Martins, C.; Ribeiro, D.; Trigueiros, N.; Szczepek, A.J.; Caria, H.; Hoare, D.J.; Paço, J.; Borrego, L.M. Tinnitus, Hearing Loss and Inflammatory Processes in an Older Portuguese Population. Int. J. Audiol. 2020, 59, 323–332.
- Shaftel, S.S.; Griffin, W.S.T.; Kerry, K.M. The Role of Interleukin-1 in Neuroinflammation and Alzheimer Disease: An Evolving Perspective. J. Neuroinflamm. 2008, 5, 7.
- Eriksson, C.; Van Dam, A.M.; Lucassen, P.J.; Bol, J.G.J.M.; Winblad, B.; Schultzberg, M. Immunohistochemical Localization of Interleukin-1β, Interleukin-1 Receptor Antagonist and Interleukin-1β Converting Enzyme/Caspase-1 in the Rat Brain after Peripheral Administration of Kainic Acid. Neuroscience 1999, 93, 915–930.
- Dinarello, C.A.; Thompson, R.C. Blocking IL-1: Interleukin 1 Receptor Antagonist in Vivo and in Vitro. Immunol. Today 1991, 12, 404–410.
- Hwang, J.-H.; Chen, J.-C.; Yang, S.-Y.; Wang, M.-F.; Chan, Y.-C. Expression of Tumor Necrosis Factor-(Alpha) and Interleukin-1(Beta) Genes in the Cochlea and Inferior Colliculus in Salicylate-Induced Tinnitus. J. Neuroinflamm. 2011, 8, 2–7.
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte Activation and Reactive Gliosis—A New Target in Stroke? Neurosci. Lett. 2019, 689, 45–55.
- Zeng, Z.; Roussakis, A.A.; Lao-Kaim, N.P.; Piccini, P. Astrocytes in Parkinson’s Disease: From Preclinical Assays to in Vivo Imaging and Therapeutic Probes. Neurobiol. Aging 2020, 95, 264–270.
- Kane, C.J.M.; Drew, P.D. Neuroinflammatory Contribution of Microglia and Astrocytes in Fetal Alcohol Spectrum Disorders. J. Neurosci. Res. 2020, 99, 1973–1985.
- Fang, L.; Fu, Y.; Zhang, T.-Y. Salicylate-Induced Hearing Loss Trigger Structural Synaptic Modifications in the Ventral Cochlear Nucleus of Rats via Medial Olivocochlear (MOC) Feedback Circuit. Neurochem. Res. 2016, 41, 1343–1353.
- Smith, L.; Gross, J.; Morest, D.K. Fibroblast Growth Factors (FGFs) in the Cochlear Nucleus of the Adult Mouse Following Acoustic Overstimulation. Hear. Res. 2002, 169, 1–12.
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-Mediated Astrocyte-Neuron Signalling. Nature 1994, 369, 744–747.
- Fellin, T.; Pascual, O.; Gobbo, S.; Pozzan, T.; Haydon, P.G.; Carmignoto, G. Neuronal Synchrony Mediated by Astrocytic Glutamate through Activation of Extrasynaptic NMDA Receptors. Neuron 2004, 43, 729–743.
- Park, K.M.; Bowers, W.J. Tumor Necrosis Factor-Alpha Mediated Signaling in Neuronal Homeostasis and Dysfunction. Cell Signal. 2010, 22, 977–983.
- Chrbolka, P.; Alušík, Š.; Kalátová, D.; Paluch, Z. Increased Platelet Activity in Tinnitus Patients. Neuroendocrinol. Lett. 2020, 41, 102–106.
- Chen, Y.; Zhong, H.; Zhao, Y.; Luo, X.; Gao, W. Role of Platelet Biomarkers in Inflammatory Response. Biomark. Res. 2020, 8, 2–8.
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging Roles for Platelets as Immune and Inflammatory Cells. Blood 2014, 123, 2759–2767.
- Aggrey, A.A.; Srivastava, K.; Ture, S.; Field, D.J.; Morrell, C.N. Platelet Induction of the Acute-Phase Response Is Protective in Murine Experimental Cerebral Malaria. J. Immunol. 2013, 190, 4685–4691.
- Kemal, O.; Müderris, T.; Başar, F.; Kutlar, G.; Gül, F. Prognostic Value of Mean Platelet Volume on Tinnitus. J. Laryngol. Otol. 2016, 130, 162–165.
- Düzenli, U.; Bozan, N.; Aslan, M.; Özkan, H.; Turan, M.; Kıroğlu, A.F. A Retrospective Analysis of Haemotologic Parameters in Patients with Bilateral Tinnitus. East. J. Med. 2018, 23, 264–268.
- Ulusoy, B.; Bozdemir, K.; Akyol, M.; Mişe, H.I.; Kutluhan, A.; Korkmaz, M.H. Investigation of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio and Mean Platelet Volume in Patients with Tinnitus. J. Laryngol. Otol. 2018, 132, 129–132.
- Behari, M.; Shrivastava, M. Role of Platelets in Neurodegenerative Diseases: A Universal Pathophysiology. Int. J. Neurosci. 2013, 123, 287–299.
- Eggermont, J.J. Tinnitus: Neurobiological Substrates. Drug Discov. Today 2005, 10, 1283–1290.
- Guitton, M.J.; Caston, J.; Ruel, J.; Johnson, R.M.; Pujol, R.; Puel, J.L. Salicylate Induces Tinnitus through Activation of Cochlear NMDA Receptors. J. Neurosci. 2003, 23, 3944–3952.
- Hwang, J.-H.; Chen, J.-C.; Yang, S.-Y.; Wang, M.-F.; Liu, T.-C.; Chan, Y.-C. Expression of COX-2 and NMDA Receptor Genes at the Cochlea and Midbrain in Salicylate-Induced Tinnitus. Laryngoscope 2011, 121, 361–364.
- Furukawa, K.; Mattson, M.P. The Transcription Factor NF-ΚB Mediates Increases in Calcium Currents and Decreases in NMDA- and AMPA/Kainate-Induced Currents Induced by Tumor Necrosis Factor-α in Hippocampal Neurons. J. Neurochem. 1998, 70, 1876–1886.
- Jara, J.H.; Singh, B.B.; Floden, A.M.; Combs, C.K. Tumor Necrosis Factor Alpha Stimulates NMDA Receptor Activity in Mouse Cortical Neurons Resulting in ERK-Dependent Death. J. Neurochem. 2007, 100, 1407–1420.
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine Mechanisms of Central Sensitization: Distinct and Overlapping Role of Interleukin-1β, Interleukin-6, and Tumor Necrosis Factor-α in Regulating Synaptic and Neuronal Activity in the Superficial Spinal Cord. J. Neurosci. 2008, 28, 5189–5194.
- Wheeler, D.; Knapp, E.; Bandaru, V.V.R.; Wang, Y.; Knorr, D.; Poirier, C.; Mattson, M.P.; Geiger, J.D.; Haughey, N.J. Tumor Necrosis Factor-α-Induced Neutral Sphingomyelinase-2 Modulates Synaptic Plasticity by Controlling the Membrane Insertion of NMDA Receptors. J. Neurochem. 2009, 109, 1237–1249.
- Liu, T.; Jiang, C.Y.; Fujita, T.; Luo, S.W.; Kumamoto, E. Enhancement by Interleukin-1β of AMPA and NMDA Receptor-Mediated Currents in Adult Rat Spinal Superficial Dorsal Horn Neurons. Mol. Pain 2013, 9, 1.
- Wang, H.T.; Luo, B.; Zhou, K.Q.; Xu, T.L.; Chen, L. Sodium Salicylate Reduces Inhibitory Postsynaptic Currents in Neurons of Rat Auditory Cortex. Hear. Res. 2006, 215, 77–83.
- Jin, Y.; Luo, B.; Su, Y.Y.; Wang, X.X.; Chen, L.; Wang, M.; Wang, W.W.; Chen, L. Sodium Salicylate Suppresses GABAergic Inhibitory Activity in Neurons of Rodent Dorsal Raphe Nucleus. PLoS ONE 2015, 10, e0126956.
- Brozoski, T.J.; Spires, T.J.D.; Bauer, C.A. Vigabatrin, a GABA Transaminase Inhibitor, Reversibly Eliminates Tinnitus in an Animal Model. JARO J. Assoc. Res. Otolaryngol. 2007, 8, 105–118.
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential Regulation of AMPA Receptor and GABA Receptor Trafficking by Tumor Necrosis Factor-α. J. Neurosci. 2005, 25, 3219–3228.
- Gerrow, K.; Triller, A. Synaptic Stability and Plasticity in a Floating World. Curr. Opin. Neurobiol. 2010, 20, 631–639.
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive Plasticity in Tinnitus-Triggers, Mechanisms and Treatment. Nat. Rev. Neurol. 2016, 12, 150–160.
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G.; et al. Tumor Necrosis Factor and Interleukin-1β Modulate Synaptic Plasticity during Neuroinflammation. Neural Plast. 2018, 2018, 8430123.
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A Multifaceted Regulator of Neuronal Plasticity and Protection. J. Neurochem. 2011, 116, 1–9.
- Vezzani, A.; Viviani, B. Neuromodulatory Properties of Inflammatory Cytokines and Their Impact on Neuronal Excitability. Neuropharmacology 2015, 96, 70–82.
- Roberts, L.E. Neural Plasticity and Its Initiating Conditions in Tinnitus. HNO 2018, 66, 172–178.
- Wu, C.; Stefanescu, R.A.; Martel, D.T.; Shore, S.E. Tinnitus: Maladaptive Auditory-Somatosensory Plasticity. Hear. Res. 2016, 334, 20–29.
- Elarbed, A.; Fackrell, K.; Baguley, D.M.; Hoare, D.J. Tinnitus and Stress in Adults: A Scoping Review. Int. J. Audiol. 2021, 60, 171–182.
- Baigi, A.; Oden, A.; Almlid-Larsen, V.; Barrenäs, M.L.; Holgers, K.M. Tinnitus in the General Population with a Focus on Noise and Stress: A Public Health Study. Ear Hear. 2011, 32, 787–789.
- Rohleder, N. Stress and Inflammation—The Need to Address the Gap in the Transition between Acute and Chronic Stress Effects. Psychoneuroendocrinology 2019, 105, 164–171.
- Stolzberg, D.; Salvi, R.J.; Allman, B.L. Salicylate Toxicity Model of Tinnitus. Front. Syst. Neurosci. 2012, 6, 28.
- Koops, E.A.; Eggermont, J.J. The Thalamus and Tinnitus: Bridging the Gap between Animal Data and Findings in Humans. Hear. Res. 2021, 407, 108280.
- Hwang, J.-H.; Huang, D.C.-W.; Lu, Y.-C.; Yang, W.-S.; Liu, T.-C. Effects of Tumor Necrosis Factor Blocker on Salicylate-Induced Tinnitus in Mice. Int. Tinnitus J. 2017, 21, 24–29.
- Manohar, S.; Dahar, K.; Adler, H.J.; Dalian, D.; Salvi, R. Noise-Induced Hearing Loss: Neuropathic Pain via Ntrk1 Signaling. Mol. Cell. Neurosci. 2016, 75, 101–112.
- Bayraktar, C.; Taşolar, S. Relationship between Increased Carotid Artery Stiffness and Idiopathic Subjective Tinnitus. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2125–2130.
- Yildiz, S.; Karaca, H.; Toros, S.Z. Mean Platelet Volume and Neutrophil to Lymphocyte Ratio in Patients with Tinnitus: A Case-Control Study. Braz. J. Otorhinolaryngol. 2020.
- Savastano, M.; Celadin, M.; Pittoni, M.; Plebani, M.; Marioni, G. Western Blot Immunoassay for HSP-70 Antibodies in Idiopathic Tinnitus: A Preliminary Report. Ann. Otol. Rhinol. Laryngol. 2006, 115, 243–246.
- Çeçen, A.; Kemal, Ö.; Yildirim, U.; Kavaz, E.; Terzi, Ö. The Clinical and Prognostic Value of the Neutrophil Lymphocyte Ratio, the Platelet Lymphocyte Ratio and Mean Platelet Volume in Tinnitus Patients. J. Exp. Clin. Med. 2021, 38, 251–254.
- Ozbay, I.; Kahraman, C.; Balikci, H.H.; Kucur, C.; Kahraman, N.K.; Ozkaya, D.P.; Oghan, F. Neutrophil-to-Lymphocyte Ratio in Patients with Severe Tinnitus: Prospective, Controlled Clinical Study. J. Laryngol. Otol. 2015, 129, 544–547.
- Weber, C.; Arck, P.; Mazurek, B.; Klapp, B.F. Impact of a Relaxation Training on Psychometric and Immunologic Parameters in Tinnitus Sufferers. J. Psychosom. Res. 2002, 52, 29–33.
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Res. Bull. 2012, 87, 10–20.
- Ledeboer, A.; Sloane, E.M.; Milligan, E.D.; Frank, M.G.; Mahony, J.H.; Maier, S.F.; Watkins, L.R. Minocycline Attenuates Mechanical Allodynia and Proinflammatory Cytokine Expression in Rat Models of Pain Facilitation. Pain 2005, 115, 71–83.
