Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Joana Oliveira.
Phenolic compounds are secondary metabolites found in plants. They can be found in high amounts in our diet due to their presence in fruits, vegetables, cereals, chocolate, and beverages such as, coffee, tea, beer, cider, and wine.
- polyphenols
- glucose
- diabetes
1. Phytochemical Compounds
Phenolic compounds are secondary metabolites found in plants. They can be found in high amounts in our diet due to their presence in fruits, vegetables, cereals, chocolate, and beverages such as, coffee, tea, beer, cider, and wine [34,35,36,37,38,39]. These compounds are responsible for cellular growth and the regulation of fruit maturation. In addition, they are involved in the defense against pathogens as well as ultraviolet radiation [40]. This is, in part, due to their ability to react directly with substances in bacterial cells, preventing them from growing, as well as to have inhibitory capacity for some important enzymes. Phenolic compounds can also modulate protein regulation and capture or trap metals and substrates, thus interfering with bacterial growth [41,42].
Phenolic compounds may contribute to bitterness, astringency, color, and oxidative stability in food. Their daily intake can reach 900 mg/day in the general population, with values varying according to different dietary and lifestyle patterns [43]. For instance, the consumption of 20 g of dark chocolate can yield to an intake of more than 600 mg of phenolic compounds [38]. This is especially true for oligomeric and polymeric flavanols [44]. Countries where the population has a moderate consumption of coffee and tea can have a substantial increase in the phenolic compound intake by more than 250 mg and 40 mg per 100 mL, respectively [37,45]. Moreover, the inclusion of red wine in the diet can also yield the ingestion of more than 200 mg of phenolic compounds per cup, depending on the wine quality, age, grape variety, winemaking process, etc. [39]. However, the phenolic compound composition may not be the same. It may depend on its original natural source. Their bioavailability may not be the same. Different classes of these compounds may present different bioactivities.
2. Structure, Food Sources, Bioavailability, and Metabolism
From a structural point of view, polyphenols are divided into two main groups, non-flavonoids, and flavonoids, as depicted in Figure 1. Non-flavonoids comprise different families of compounds with dissimilar structural features, including simple phenols, phenolic acids (benzoic and hydroxycinnamic acids), stilbenes (e.g., resveratrol), hydrolysable tannins, and lignans and polymeric lignins (Figure 1a). On the other hand, flavonoids are composed of a great diversity of families of compounds with a similar structural pattern, all of which have a flavanic core. The flavanic core is composed of two aromatic rings, A and B, that are linked through a heterocyclic pyranic ring C. The differences observed between each family of flavonoids is in the hydroxylation pattern of the rings and in the unsaturation degree of ring C [46]. Flavonoids comprise flavan-3-ols, flavonols, flavones, flavanones, flavanonols, isoflavones, chalcones, and anthocyanins (Figure 1b).
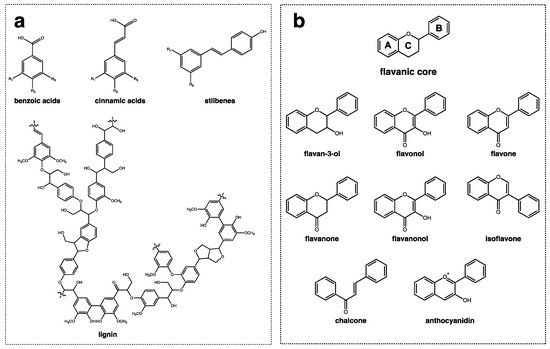
 Figure 1. The general structure of phenolic compounds with (a) non-flavonoid compounds comprising benzoic, cinnamic acids, stilbenes, and lignins; and (b) flavonoid compounds represented by the flavanic core that is composed by two aromatic rings ((A) and (B)) and a pyranic ring (C) and their families of compounds: flavan-3-ol, flavonols, flavones, flavanones, flavanonols, isoflavones, chalcones, and anthocyanidins.
Figure 1. The general structure of phenolic compounds with (a) non-flavonoid compounds comprising benzoic, cinnamic acids, stilbenes, and lignins; and (b) flavonoid compounds represented by the flavanic core that is composed by two aromatic rings ((A) and (B)) and a pyranic ring (C) and their families of compounds: flavan-3-ol, flavonols, flavones, flavanones, flavanonols, isoflavones, chalcones, and anthocyanidins.Phenolic acids, especially hydroxycinnamic acids and non-flavonoids, are one of the most abundant classes of phenolic compounds found in nature, and this includes o-, m-, and p-coumaric acid, sinapic acid, ferulic acid, and caffeic acid [47,48]. These phenolic compounds can be present as free carboxylic acids or as esters of tartaric and quinic acids, flavonoids, and carbohydrates [48]. Hydroxycinnamic acids are widely distributed in plant families, with several species being part of the diet or processed into beverages, including fruits, vegetables, and grains [48,49]. Regarding stilbenes, resveratrol is one of the most well-known compounds. It is produced by plants in response to injury or attack by pathogens such as bacteria or fungi. It plays an important role in the defense against stress-inducing conditions. Resveratrol has been found in some nutritional foods such as white squash, the roots of Polygonum cuspidatum, grapes, bilberries, blueberries, cranberries, and peanuts [50,51,52]. However, the amount of resveratrol present in these foods is generally low, ranging from 0.02 to 3 mg per 100 g (fresh weight) [53,54].
On the other hand, proanthocyanidins are reported as one of the major fraction of the total flavonoids ingested in Western diets [44]. These are polymers of flavan-3-ol widely present in vegetables, plant skins, seeds, flowers, fruits, and nuts [55]. Although results are scarce reporting the daily human consumption of these flavan-3-ol polymers, one study has described that the mean daily intake of proanthocyanidins, especially oligomers and polymers, in adults of the United States of America (USA)’s population was estimated to 57.7 mg/person [44]. On the other, the younger (2–5 year-old) and elderly (>60 year-old) populations were the groups with the highest consumption of proanthocyanidins, 68.2 mg/person and 70.8 mg/person, respectively, due to their higher ingestion of fruits and vegetables [44].
Anthocyanins are water-soluble pigments found in many fruits, flowers, and some legumes and are responsible for the red, violet, and blue colors found in nature. Structurally, they are characterized as glycosides of the flavylium cation polyhydroxylated and/or methoxylated. More than 500 anthocyanins have already been identified in nature, although cyanidin-3-O-glucoside represents around 50% of the anthocyanins present in red fruits [56]. Blackberries and blackcurrants contain up to 2–4 g per kg (fresh weight) of anthocyanins that are present mainly in the skin [56]. Red grapes can contain a similar content of anthocyanins (around 2.5 g per kg) and their processed beverages such as red wines, especially young wines, can contain up to 1 g per liter, with this amount varying according to the grape variety, the wine production methodology, the year of production, etc. [57,58].
Therefore, phenolic compounds, including flavonoids, namely proanthocyanidins and anthocyanins, and non-flavonoids such as phenolic acids and resveratrol, are a large and heterogeneous group of phytochemicals that are present in and are an important part of plant-based foods, such as tea, coffee, wine, cocoa, cereal grains, soy, fruits, and berries, which are part of our daily diet. Although there are no data regarding the amount of phenolic compounds needed to be ingested to have an healthy diet, the World Health Organization and Food and Agriculture Organization of the United Nations (WHO/FAO) report recommends a population-wide intake goal of 400 g of edible fruit and vegetables per day for the prevention of non-communicable diseases [59]. This amount of fruit and vegetables corresponds to a regular dietary intake of polyphenols of approximately 1–2 g per day [60].
Epidemiological evidence reporting on the ability of phenolic compounds to modulate or prevent T2D are scarce, and most date earlier than 2015 [33]. Generally, epidemiological studies show partial protection against the development of T2D due to the consumption of whole fruits for long periods of time. According to animal studies, the direct acute effect of (poly)phenols on postprandial glycemic response is relatively small, with a more modest effect on digestion and sugar absorption within the gut [61]. The notable effects from citrus (poly)phenol metabolites are on post-absorptive processes, such as the modulation of hepatic glucose metabolism and insulin sensitivity in target tissues [61].
On the other hand, and considering some of the most recent examples, there are some promising results to highlight. Diets naturally rich in phenolic compounds, from decaffeinated green tea, coffee, dark chocolate, blueberry jam, extra-virgin olive oil, and polyphenol-rich vegetables (artichokes, onions, spinach, and rocket), reduced plasma glucose, observed when measuring the total AUC (p = 0.038), likely by increasing early insulin secretion (AUC 0–30 min, p = 0.048) and insulin sensitivity in people at high cardiometabolic risk who had a large waist circumference [62].
In a double-blind, 8-week randomized controlled study involving 80 patients with diabetes, Brazilian green propolis (226.8 mg/day) rich in phenolic compounds was found to prevent worsening of blood uric acid and the estimated glomerular filtration rate [63]. In a controlled crossover trial, 11 healthy fasted volunteers consumed 300 mL of either light (LIR) or dark (DAR) roasted coffee, or water, followed by an oral glucose tolerance test (OGTT) [64]. Two coffees with dissimilar chlorogenic acid contents did not differentially affect glucose or insulin responses during an OGTT, but both raised insulin concentrations and reduced the insulin sensitivity index (ISI) compared with water, with no difference between them [64]. Daily consumption of polyphenol-rich extra-virgin olive oil improved metabolic control (significant reduction in fasting plasma glucose (p = 0.023), HbA1c (p = 0.039) levels, BMI (p = 0.012), and body weight (p = 0.012)), as well as the circulating inflammatory adipokine profile, in overweight patients with diabetes that were not being treated with insulin [65].
Guo and co-authors showed that risk of T2D development was decreased by 5%, with a 7.5 mg/day increment of dietary anthocyanin intake or with a 17 g/day increase in intake of blackberries, blueberries, raspberries, strawberries, or other berries [66]. For isoflavones, higher daidzein and genistein concentrations were associated with a lowered risk of diabetes in Korean and USA women, as well as in middle-aged and elderly Chinese volunteers [67]. In the framework of the described studies, there is clear evidence that the way phenolic compounds are consumed, their amount, and whether they are consumed acutely or chronically (short or long periods) does indeed affect their bioaccessibility, in turn also affecting their bioavailability. These are points of special relevance that deserve to be studied with more precision to drive research towards more accurate findings.
Special focus has recently been given to phenolic compounds that are easily extracted from plants with water or hydro-alcoholic/organic solvents, as well as to other factors influencing the bioavailability of free phenolic compounds [68]. Although the first mechanism proposed for the action of phenolic compounds in the human body is based on their direct antioxidant properties, these effects are no longer considered so relevant in vivo. The reason for this is that phenolic compounds are subjected to an intense metabolism due to the gastrointestinal digestion process. The native forms of these compounds present in food do not reach the target tissues in sufficiently high concentrations to have a significant effect in terms of neutralizing free radicals. Phenolic compounds are mostly like sugars, (glucose, galactose, rhamnose, xylose, rutinose, arabinopyranose, and arabinofuranose) O-conjugates at the C2 (chalcones), C3 (flavonols, anthocyanidins, and flavan-3-ols), or C7 (flavanones, flavones, and isoflavones) positions. These and other modifications (methylations and galloylation) add extra structural stability to phenolic compounds, reducing their ability to reach systemic circulation. Furthermore, their chemical structure determines whether these compounds are hydrolyzed and modified by enzymes before absorption, such as lactase phlorizin hydrolase (LPH) (at the enterocyte membrane) or β-glucosidase (CBG) (cytosolic, for polar glycosides). Their transport to liver via the portal vein by active, passive, or facilitated transportation can only occur after this metabolization process [69]. A major challenge associated with the bioactivity of phenolic compounds is their lack of bioavailability in vivo, which is primarily the result of coupled metabolic activities of conjugating enzymes and efflux transporters. Only 1–10% of the total phenolic compounds ingested are absorbed by the small intestine, largely due to the extensive metabolism they have to undergo [70]. A large portion of phenolic compounds are enzymatically transformed by methylation, glucuronidation, or sulfation in the liver, but also in the kidneys, intestine, and stomach [71]. Therefore, it is expected that these processes represent a significant barrier to the oral bioavailability of phenolic compounds and facilitates their biliary and urinary elimination by increasing their hydrophilicity. For example, anthocyanins are absorbed with poor efficiency in their parent forms and are therefore rapidly metabolized and eliminated [72]. Glucuronides and methyl conjugates of anthocyanins were identified in human urine and plasma by liquid chromatography coupled with mass spectrometry analysis after blackberry juice intake [73]. Furthermore, other metabolites were identified as degradation products, phenolic, hippuric, phenylacetic, and phenylpropenoic acids, which were present in the circulation for ≤48 h after ingestion [72].
In recent years, progress has been made on the identification of possible biochemical and molecular mechanisms of action of phenolic compound-derived metabolites, which depend fundamentally on their ingested doses through the food [43] and their effective bioavailability, or the amount that reaches the target tissue.
Importantly, phenolic compounds are used to slow starch digestion and in the transport of simple sugars across the intestinal epithelia, thereby reducing the plasma blood glucose spike after a meal. These effects are achieved through the inhibition of amylases, glucosidases, and glucose transporters present in the gastrointestinal tract and the brush boarder membranes [74]. The extent of inhibition by phenolic compounds is dependent on their molecular structure, intake doses, and the food matrix. For example, more polymerized procyanidins have more interaction sites with proteins and therefore will inhibit more efficiently α-amylase activity [75]. Likewise, Xiao and coworkers concluded that milk did not influence the oral relative bioavailability of pelargonidin anthocyanins under meal conditions. However, the oral relative bioavailability of pelargonidin anthocyanins was reduced by milk, by ~50% under fasting conditions (p < 0.05) [76]. Similarly, when these extracts of phenolic compounds are incorporated into food systems, they can interact with other biopolymers (carbohydrates, proteins, lipids) in the food matrix. This leads to lower inhibition of digestion and/or glucose transporters, compared to when they are ingested as an extract or in pure compound forms without additional matrix biomolecules, as often observed in most in vitro cell systems [77].
Greater consumption of specific whole fruits, particularly blueberries, grapes, and apples, has been significantly associated with a lower risk of T2D. On the other hand, increased consumption of fruit juice, instead of whole fruits (particularly from blueberries, grapes, and apples), seems to lead to an increased risk of T2D development, as observed in a study where 187,382 health professionals were free of major chronic diseases at baseline [78]. However, in a double-blind crossover acute trial in 34 healthy adult subjects, it was demonstrated that apple drinks enriched with phlorizin-rich apple extract reduced plasma glucose levels [79]. Another double-blind crossover trial with 25 healthy adult subjects showed that apple phenolic compounds reduced postprandial insulin, C-Peptide, and the insulin area under the curve at 30 min after a high-carbohydrate meal [80]. Recent findings suggest that the daily consumption of a cranberry beverage over 8 weeks may not have an impact on insulin sensitivity but it may be beneficial to lower triglycerides and change oxidative stress biomarkers including 8-isoprostane, a biomarker of lipid peroxidation and nitrate for nitric oxide production, in obese individuals with a proinflammatory state, with C-reactive protein levels > 4 mg/L [81].
Moreover, it is still not clear how the delivery of phenolic compounds in whole plant foods or the delivery of individual functional ingredients such as supplements impact the alteration of T2D risk markers. Few studies have been consistently performed to ascertain their functional specificity or their specific molecular effects. Random forms of consumption of these substances may be associated with the contradictory effects sometimes described. Considering the way these compounds are ingested, fruits are commonly consumed worldwide in both fresh and processed forms, especially as juices. While fruit consumption is perceived as beneficial for long-term health, the effects of fruit juices are more controversial and are associated with a high intrinsic sugar content. On the other hand, both forms are rich in bioactive compounds other than macro- and micronutrients. Furthermore, the bioaccessibility of phenolic compounds may importantly affect health outcomes.
Generally, phenolic compounds can be found in plant matrices in two forms, extractable and bound (Figure 2). Extractable phenolic compounds do not interact with other plant macromolecules and can be easily extracted after plant tissue disruption. On the other hand, bound phenolic compounds can be found cross-linked to cell wall macromolecules, such as cellulose, pectin, hemicellulose, lignin, and rod-shaped structural proteins [82] or can be physically entrapped within the food matrix [83]. In addition, the bound phenolic compounds also include all those phenolic compounds that are not extracted with water or mixtures with the most common organic solvents, such as ethanol, methanol, or acetone, and therefore are not assessed by the most used techniques for phenolic compounds analysis.
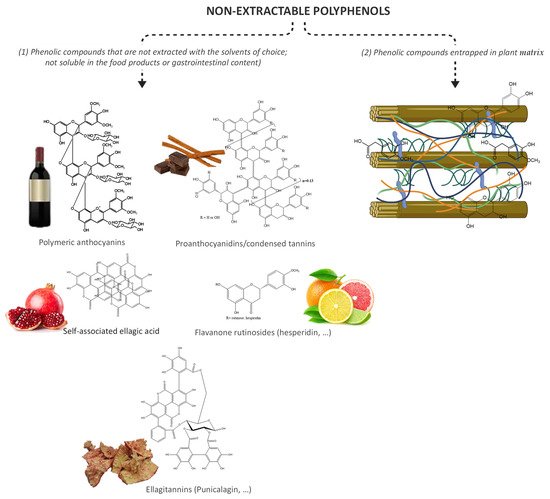
 Figure 2. Non-extractable polyphenols, including (1) non-bioavailable polyphenols and (2) complex structures composed by cellulose microfibrils, hemicelluloses (xyloglucans, xylans (arabinoxylans, glucuronoxylan, and glucuronoarabinoxylan), glucomannans, and mixed-linkage glucans), pectins, lignins, bound polyphenols, and proteins.
Figure 2. Non-extractable polyphenols, including (1) non-bioavailable polyphenols and (2) complex structures composed by cellulose microfibrils, hemicelluloses (xyloglucans, xylans (arabinoxylans, glucuronoxylan, and glucuronoarabinoxylan), glucomannans, and mixed-linkage glucans), pectins, lignins, bound polyphenols, and proteins.Hence, there has been considerable interest in understanding and exploiting the functions and health benefits of whole food systems, as these conjugates may have improved health attributes that may not be achieved using individual components, such as a great impact on microbiota modulation. For example, in a randomized, double-blinded, placebo-controlled, cross-over trial involving 22 healthy adult males, under daily supplementation of 500 mg of quercetin, no influence on the fasting glucose was observed [84]. However, the effectiveness of onion to ameliorate hyperglycemia and insulin resistance was observed in a parallel-design, randomized, triple-blind, controlled clinical trial, conducted on 56 eligible breast cancer patients (aged 30–63 years) [85]. In a meta-analysis, an increase in the consumption of fruit, especially berries, vegetables, and their fiber, was associated with a reduced risk of T2D development [86].
Besides being a major part of dietary phenolic compounds, non-extractable phenolic compound complexes have been very poorly studied [87]. These complexes that are undigested in the upper digestive tract reach the colon, where the fermentation process is responsible for bacterial growth modulation [88] (Figure 3). Importantly, bacterial fermentation processes contribute to the release of free phenolic compounds, either as intact compounds or their derived metabolites (hydroxyphenylacetic and hydroxyphenylvaleric acids or urolithin, among others). These compounds can modulate microbiota inducing prebiotic-like effects on bacteria, while also being metabolized by intestinal bacteria into specific bioavailable metabolites [89]. Furthermore, there is evidence that intact parent (poly)phenols such as green tea catechins can inhibit glucose uptake in the gut. On the other hand, there is evidence that phenolic compound gut microbial metabolites, such as isovanillic acid 3-O-sulfate, a metabolite from the microbiome found in blood, primarily derived from consumed cyanidin-3-O-glucoside, could indeed stimulate glucose uptake in tissues by glucose transporter GLUT4- through PI3K-dependent mechanisms, as observed in the differentiated human skeletal muscle myoblast line, LHCN-M2 [90].
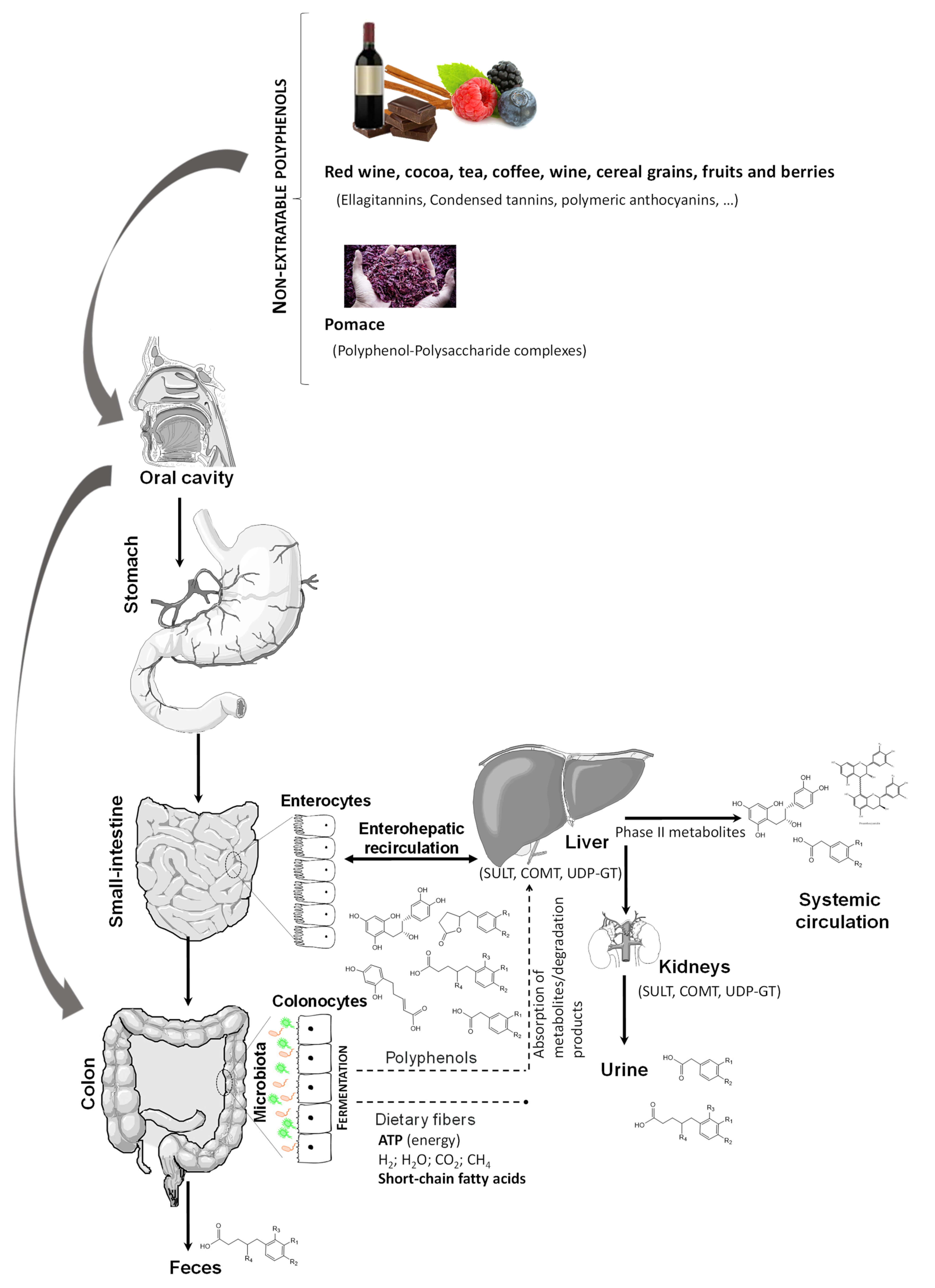 Figure 3. Oral ingestion and gut microbiota catabolism of non-extractable polyphenols. SULT: Sulfotransferases; UDP-GT: Uridine 5′-diphospho-glucuronosyltransferase; COMT: Catechol-O-methyltransferase.
Figure 3. Oral ingestion and gut microbiota catabolism of non-extractable polyphenols. SULT: Sulfotransferases; UDP-GT: Uridine 5′-diphospho-glucuronosyltransferase; COMT: Catechol-O-methyltransferase.These different but complementary effects of parent compounds and microbial products are a promising line of research and could conceivably lead to synergistic effects between the parent compound and its microbial degradation products. However, it is important to keep in mind that changes in the gut microbiota composition and functionality among individuals may affect phenolic compound metabolism and therefore their health effects. For instance, gut dysbiosis induced by a high-fat (HF) diet can dramatically change the type and amount of endogenous and exogenous metabolites formed in the gut [91]. However, blackberry anthocyanin-rich extract can modulate gut microbiota composition and counteract some of the features of HF diet-induced dysbiosis.
Importantly, it should be highlighted that in vitro and perhaps also in vivo assays using pure phenolic compounds are probably physiologically irrelevant. The real environment, centered in a meal, which should be composed of a balanced amount of dietary phenolic compounds at physiological doses (between 1–10% of the ingested dose; if considering the Mediterranean diet, this should be around 1 g/day of total polyphenols), with included dietary fibers and other indigestible substrates [92]. Research supports the health benefits of a Mediterranean-style eating pattern that includes a “colorful” plate with several varieties of different foods, including various macro- and micro-nutrients. It is the combination of these foods that appear to confer most protection against disease, as the benefit is not as strongly observed when looking at single foods or pure nutrients [93].
Meanwhile, it should be highlighted that non-extractable phenolic compounds can have a fundamental role for nutritionists and physicians, since they are classified as macromolecular antioxidants [94]. Unlike the dietary antioxidants that are absorbed in the small intestine, macromolecular antioxidants reach the colon intact and, through the action of gut microbiota, may release to the colon simpler phenolic compounds. In combination, these antioxidants can help each other in promoting different antioxidant mechanisms. This synergy may support the need of a diet diversification to achieve the best health protection effects, as recommended by the Mediterranean diet.
Furthermore, phenolic compounds complexed with carbohydrates may act as a delivery nanostructure. These structures may protect the antioxidant from degradation and metabolism during gastro-intestinal passage and by enterohepatic recirculation ensuring the release at the bloodstream of “free” polyphenols. These “free” polyphenols (structures less polymerized or not conjugated with other types of molecules, such as sugars) suffer the action of gut microbiota, giving rise to metabolites that are much better absorbed reaching significant concentrations in plasma, which may persist for 3–4 days. Therefore, the intake of food sources rich in non-extractable phenolic compounds could be a strategy to reach a sustained systemic anti-inflammatory and antioxidant status in the human body thanks to the constant production of bioactive microbial metabolites [95]. This has been, so far, the first mechanism proposed for the action of phenolic compounds in the human body and their direct antioxidant properties, which, in the context of this health preventive picture, seem to be more than ever relevant.
In addition, recent findings suggest that some phytochemicals, such as sulforaphane, exhibit an hormetic effect in cells at low doses, activating signaling pathways that result in the increased expression of genes encoding cytoprotective proteins and antioxidant enzymes [96,97]. As such, the relatively small doses of phytochemicals ingested by humans are not toxic and instead induce mild cellular stress responses. The nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway is an important cell signaling mechanism in maintaining redox homeostasis in humans [98,99,100]. The hormetic effect has, for instance, been reported for some flavonoids, including [101] luteolin, apigenin, quercetin, myricetin, rutin, naringenin, epicatechin, and genistein, and due to their antioxidant or prooxidant activity, depending on their concentrations, they contribute to redox homeostasis [98,102]. Furthermore, included in the category of “non-extractable polyphenols” are also the higher molecular weight phenolic compounds, polymeric structures, proanthocyanidins, and hydrolysable tannins. In addition, other phenolic compounds that are not complexes or oligomeric and that reach the colon almost unaltered since they are not soluble in food and in gastrointestinal content include hesperidin, due to solubility issues, and ellagic acid, as it self-associates into planar molecules, properties that greatly limit their absorption [95]. These points may explain, at least in part, the lack of evidence from clinical studies using phenolic compounds.
