Fundamentally, the process steps for pyrolysis as well as its complementing factors are significant to the general application of pyrolysis. Thus, with thermal pyrolysis being the basis of any pyrolysis process, such factors influence the process and eventually the outcome (products) in one way or the other. Inasmuch as most studies emphasise the effects of thermal pyrolysis temperature, heating rate and chemical composition of feedstock with reference to the product yield, the general essence of factors that affect pyrolysis is overwhelmingly significant. Common among other factors include the reactor type, residence time and pressure, as discussed below. These factors have continued to influence many types of research including their modification and further developments.
Various types of research over the years have depicted factors that affect plastic thermal pyrolysis, including the products of pyrolysis. Reputable among these types of research are those conducted by Jung et al.
[1] and Zhang et al.
[2], in which they investigated the influence of pyrolysis temperature stretching from 200 °C to 800 °C. Banik et al.
[3], Gai et al.
[4], Hassan et al.
[5] and Mandal et al.
[6] looked into both pyrolysis temperature and feedstock as pyrolysis factors, and Zhao
[7] showcased the effect of pyrolysis temperature, residence time and heating rate associated with a range of waste plastics, such as polypropylene (PP), polycarbonate (PC) and high impact polystyrene (HIPS). As mentioned earlier, the significance of the factors affecting plastic pyrolysis glues to and targets the desired yields and the pyrolysis process in general. These factors influence the molecular structure of pyrolytic products (yields). The major factors include the chemical composition of the feedstock under investigation, reactor type, cracking or decomposition temperature and heating rate, residence time and operating pressure.
1.1. Chemical Composition of the Feedstock
The major component of this uniqueness is characteristic of the chemical structure of the feedstock. In research conducted by Sun et al.
[8], it was established that the proportion of hydrogen (H), one of two of the major constituents of plastics, shrank in all kinds of biochars, triggered by the elimination of moisture content and thus, dehydrogenation. Furthermore, upon the completion of the pyrolysis, the content of nitrogen (N) in coffee grounds, shrunk, but an increase was observed in corn stalk. Similarly, for PVC feedstock, the composition is comprised of HCl/vinyl chloride, an impurity, and this is a source for the pyrolytic yield of (H) and other fuel energies. Kim
[9] pyrolytically experimented with PVC feedstock to produce bioenergy through both a thermodynamic and kinetics study that revealed the production of bioenergy as a promising way to go.
Buekens
[10] highlighted three groups of plastics. From these groups, the first, a catalytic or peroxide-initiated polymerisation of monomer(s), happens to be among the most desirable groups of plastics for the purpose of feedstocks for pyrolysis
[10]. Thus, the quality of plastic-derived fuels is dependent largely on the process and the feedstock. The diesel-range products in LDPE-derived fuels constitute similar linear alkane chains as those found in conventional diesel. In addition to this, additives otherwise referred to as impurities, are associated with these feedstocks as contaminants, leading to a negative influence on the feedstock performance, and hence poor product yields. Fundamentally, the feedstock is a significant factor, if not the most significant of all, and with it (waste plastic) being chemically recycled into monomers, a mixture of chemicals or conversion into fusion gas or reducing gas called fuels or other useful products are attainable
[10] with the aid of the reactor type that may be used in the pyrolysis.
1.2. Reactor Type
The type of reactor utilised in pyrolysis is just as significant as the fuel yield
[11]. Nonetheless, as mentioned earlier, other factors are also significant to the yield percentage and fineness. In a study performed by Buekens
[10], the selection of the reactor type is due principally to technical considerations, primarily its feed and residue handling characteristics and heat transfer. The selecting of a reactor is a key approach towards the mechanics of pyrolysis and is pivotal to product delivery.
This consequently explains the rationale behind the fact that the following two steps take effect from the moment waste plastic goes into the reactor: (1) fusion of the waste plastics and (2) pyrolysis of the fused waste plastics.With reference to step one, a non-stop feeding system is permissible for controlled pyrolysis. Hence, the need for improvement on this step is feeding into the reactor using different reactors
[12][13]. Separating the above two steps fosters the minimisation of secondary product formation, such as methane, char and even liquid (polycyclic aromatic hydrocarbons, PAHs)
[14]. With the different reactors used for the experiment, including screw reactors, fluidised beds, conical spouted beds and even spouted bed reactors, solid material particles were made use of, coated with the fused plastics, thereby facilitating the heat and mass transfer between the plastics and the gas. Free-fall reactor is another notable reactor type, which Ellens
[15] utilised to produce biofuels. In this work, the performance of a modern central composite design of the experiments leads to the optimisation of the reactor. As mentioned in section 1.1, the feedstock (polymeric materials) utilised in pyrolytic processes are often subjected to pre-treatments and related procedures before they are put into the reactors and related mechanisms. Many processes proposed scenarios wherein the polymer is first scattered in a salt bath or quenched in a pool of molten polymer or wax to lessen the viscosity of the meltdown
[10]. Furthermore, other processes can recommend the utilisation of the exceptional heat transfer and combining properties of fluidised bed, thermal or even catalytic reactors. The application of an extruder is a similar practical example of this. The extruder serves as a pre-treatment mechanism that links to the reactor for an optimal pyrolysis process.
Fluidised bed reactors are among the notable reactors known in today’s chemical industry, especially regarding the processes associated with solid elements. According to Zafar
[16], fluidised bed furnaces are among the most used pyrolysis reactors in the chemical industry, as well as rotary hearth furnaces and rotary kilns. A major reason for this is the nature by which they are built, a continuous feeding system
[17], and their high heating capabilities
[18]. Pandey et al.
[19] used a fluidised bed reactor in their pyrolysis of waste plastics to produce environmentally friendly products. They highlighted that fluidised bed reactors are one of the most reputable reactors utilised in the continuous conversion process of waste plastics pitching towards operational optimisation. However, the conical spouted bed reactor has shown a more vigorous movement of solid particles than in that of bubbling fluidised bed reactors. With reference to many citations by authors, this stimulates the minimisation of the defluidisation problems of volatile sand melted plastic melting coating, influencing the fusion of the waste plastics and pyrolysis of the fused waste plastics.
1.3. Decomposition Temperature and Heating Rate
The decomposition otherwise termed cracking temperature and heating rate in pyrolysis are as significant as the process of pyrolysis itself. The temperature in this context refers to external heat/thermal application. This cracking or reaction temperature can vary due to other factors, such as the type of waste plastic feedstock and the desired product. For example, Singh and Ruj
[20] utilised a reaction temperature of 450–600 °C to yield gaseous products from PE, PP, PS, and PET as waste plastic feedstock, whereas, in the work conducted by Zafar
[16], it was 350–800 °C for bio-oil production using municipal solid wastes (MSW) as feedstock. Regarding the literature review by Gao
[21], the reaction temperature with PE is emphasised as the most vital factor that inclined the whole pyrolysis process. However, this reaction temperature can vary even for similar reactors and feedstock materials. The variance in the position of the temperature sensor of the reactors discussed in different studies is the reason for the difference in temperatures for the same reactor type and feedstock composition
[21]. Another study conducted by Buekens
[10] proved that temperature is the most vital functional factor associated with pyrolysis. Decomposition temperature establishes both the rate of thermal disintegration and the stability of feedstock and products yielded upon the completion of the reaction. This means that there must be some cracking or degradation in the first instance for pyrolysis to occur. Heat and thus, the cracking temperature are the key parameters in this process. Cracking or the decomposition temperature is the point at which the degradation of the polymers (plastic in this case) takes effect, but with varying pyrolytic impacts with respect to the state of the product. López et al.
[22] proved that temperature strongly affects the characteristics of pyrolytic liquids and to a lesser extent in gas and solid properties. With reference to a work conducted by Singh and Ruj
[20], an increment in temperature favours an increase in oil yield in plastic pyrolysis. In fact, with this rise in the oil yield, the polymeric aromatic components of the municipal plastic wastes (MPW) also increase in formation. Generally, with an increase in temperature, the oil density decreases. In a study by Mansur et al.
[23], the breakdown of the plastic materials into chemicals of low carbon chains (say C8–C9) was partial, yet significantly supported by the influence of heat and temperature. The heat and/or temperature in pyrolysis can adopt a range of low, moderate or high temperatures to produce new materials. For example, a high temperature (>600 °C) and both vacuum and yield dilution facilitate the manufacture of basic insignificant gaseous molecules
[10]. However, a low temperature (<400 °C) and expanded pressure, point to additional viscous liquid yields, elevated rates of pyrolysis, an immense coking capability and extra minor products and dehydrogenation
[10]. It is worth noting that the plastic liquefaction of oil mainly consists of cyclic chemical compounds. Additionally, cyclic compounds are known to constitute higher boiling points than their acyclic or open-chain isomers. This means if the conversion of such oil into a more refined oil or related valuable yields, further cracking is required. Hence, more heat energy and longer residence time.
It is imperative to note that the formation of char is voluminous with MPW being that the feed materials sourced from MSW consist of extra particles and related impurities
[24][25]. The study used virgin or simulated plastic waste pyrolysis for its verification. The quantity of char produced was typically low. This difference in the results can point to the presence of impurities and related contamination factors, such as additives
[24]. Wang et al.
[26] found that biochars prepared at an increased pyrolysis temperature, heighten the productions of (H) and established that the pH cushioning volume is the central component of biochar promoting fermentative (H) production. Unlike biochar, other petrochemical wastes, such as plastics in general, require a higher pyrolysis temperature to increase the degree of carbonisation
[27]. Hydrogen (H) and carbon (C) are the most predominant constituent elements of these petrochemical wastes. Additionally, aside from (H) and (C) formations, gaseous products (also constituents of H and C) are the other reputable materials acquired. Singh and Ruj [
[20]] reported the effect and formation of gaseous products with respect to the experimental tests, demonstrating that an increase in (H
2), gives rise to a temperature increment with a decrease in low molecular weight hydrocarbons (HCs). This seems to be in agreement with Buekens
[10], whose work revealed that the increase in temperature significantly influences the relative stability of a variety of products, including the kinetics and physical conditions of the reacting mixture. Nonetheless, the initial stage of pyrolytic reactions was solidly affected by the spectre of additives, such as pigments, plasticisers and stabilisers. As such, a medium temperature ranging between 400–500 °C was chosen, and the plastics were in a liquid phase, whereas ‘gas phase’ processes made way for liquid polymer films, spreading across the grains of the fluidised bed pyrolysis reactor type
[10]. These additives in the feedstock that affect the initial stage of the pyrolysis process do so not only after the decomposition of temperature and heating rate but also the residence time as well to allow complete reaction.
1.4. Residence Time
Residence time, otherwise referred to as reaction time, is another pivotal factor of plastic pyrolysis. Research has shown that primarily decomposition/reaction temperature determines the required residence time. Short residence time is believed to aid the creation of primary products, such as monomers, while the creation of more thermodynamically stable products, such as H
2, methane (CH
4), aromatics and (C) are linked to long residence times
[10]. Monomers of plastics can include but are not limited to, the organic compounds of ethylene, propylene, styrene, vinyl chloride, formaldehyde and even phenol
[28]. The formation of these monomers, including other HCs, such as propane, and n-butane is influenced by the residence time of volatiles in a reactor
[20]. As mentioned earlier, there is a strong relationship between reaction temperature and residence time. Singh and Ruj
[20] pointed out this relationship in their work, in which the effect of residence time is portrayed to be large at higher temperature process requirements, leading to the production of heavier HCs in gas and in oil than in wax, with PE as feedstock. This is among the conditions under which no carbon monoxide (CO) and/or carbon dioxide (CO
2) is noticeable, but there is certainly a noticeable increase in H
2 production. The production of CO, CO
2 and H
2 gases are associated with waste plastic pyrolysis as oxidation occurs during pyrolysis. This is referred to as oxidative pyrolysis, giving rise to the production of non-condensable gases, such as CO and CO
2. One other significant effect of this condition is the recovery time of non-condensable gases, which is perceived to be a smaller amount at increased temperatures, but with a generally increased yield upon increasing the operating temperature. This is technically in alignment with thermal pyrolysis. However, optimising this approach with the aid of a catalyst has experimentally shown that the yield of such non-condensable materials can increase exponentially. A reputable example for this scenario can be seen in a work carried out by Singh and Ruj
[20] and Gao
[21]. They revealed that with the application of a ZSM-5 catalyst, the percentage of the non-condensable gases is boosted from 17% w/w to approximately 60% w/w by adding 10% w/w NKC-5 into the PE feedstock. The residence time can be strongly identified from the positions at which the classification of pyrolysis is analysed. Technically, each of these classifications has a different definition of what residence time is. Slow pyrolysis (and batch process), which is the conventional pyrolysis, depicts residence time as the time duration from when the waste plastic begins to heat up to that when the products are attained
[10]. This means that a slow heating rate and long residence time are associated with this class of pyrolysis, and it enhances the yield of the carbonisation process, resulting in a greater yield of tar and char. With a longer residence time, a further conversion process of the primary products into secondary ones (such as light molecular weight hydrocarbons and non-condensable gases), which are more thermally stable
[29][30][31], is achievable. A long residence time is known to foster light hydrocarbon yields
[21], unlike a short residence time, in which case the volatiles minimise the formation of secondary products, such as methane, liquid and char
[13][32]. Fast, or otherwise referred to as a continuous pyrolysis process, defines this as the contact time from the point the plastic touches the hot surface until the end of the reaction
[33].
1.5. Pressure
Operating pressures influence both pyrolysis processes and their subsequent products. Operating pressures in pyrolysis can be either low or high. A low pressure (under vacuum or in the presence of inert diluent) favours the production of primary products, including monomers, whereas complex liquid fractions are associated with high pressures
[10]. With high pressure, the boiling point of pyrolytic products attains an increment. This increment fosters a pressurised environment, a condition in which heavy HCs are further pyrolysed rather than vaporised at a given operating temperature
[34]. Essentially, as explained in the work of Sato and Sakata
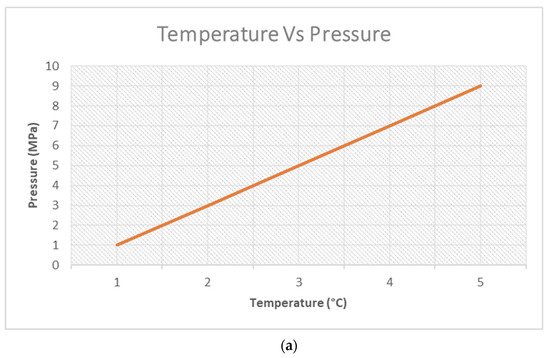
[34], more heat energy is permissible for increased HC cracking with respect to a pyrolysis system subjected to a pressurised reaction. The issue of a pressurised system is associated with high pressure, in effect. It is worth looking at
Figure 21a, which is a graphical representation of Gay Lussac’s Law, to put this into context. As such, with an increase in pressure, the system becomes compressed, implying less occupying space for the gases (non-condensable gases in this case), thereby giving rise to a reduction in their average molecular weight due to their increased speed. In the computed
Table 3 1 below, Sato and Sakata
[34] showed this effect. Furthermore, the role of temperature with respect to pressure is depicted; the relationship is directly proportional. With a temperature increase, the pressure increases (pressurised), thereby increasing the motion of the gas molecules (gas yield for the case of pyrolysis).
Figure 21.
(
a
) A graphical representation of Gay Lussac’s Law, (
b
) A schematic of Gay Lussac’s Law (adapted from
).
Table 31.
Relationship of pressure with respect to the different temperatures for gas yield (adapted from
).
| Gas Yield/wt vs. Degeneration Pressure/Mpa |
| @ 410 °C |
6.4 vs. 0.1 |
7.0 vs. 0.2 |
9.0 vs. 0.4 |
10.4 vs. 0.6 |
13.0 vs. 0.8 |
| @ 420 °C |
4.4 vs. 0.1 |
5.4 vs. 0.2 |
6.0 vs. 0.4 |
7.3 vs. 0.6 |
8.0 vs. 0.8 |
| @ 430 °C |
4.3 vs. 0.1 |
4.4 vs. 0.2 |
5.2 vs. 0.4 |
6.0 vs. 0.6 |
6.2 vs. 0.8 |
| @ 440 °C |
3.8 vs. 0.1 |
4.0 vs. 0.2 |
4.8 vs. 0.4 |
5.0 vs. 0.6 |
5.8 vs. 0.8 |
The graphical representation in
Figure 21a is a theoretical display of Gay Lussac’s Law, which asserts that the pressure of a given quantum of gas at ideal conditions held at a constant mass and volume is directly proportional to the temperature involved
[36]. In the context of pyrolysis, the heating of the system paves the way to increased temperatures. This engages the molecules of the waste plastic feedstock in an excited state, increasing their impacts on the reactor walls. Hence, a pressurised reaction system is established. This then gives rise to the increased motion of the gas molecules, as depicted in
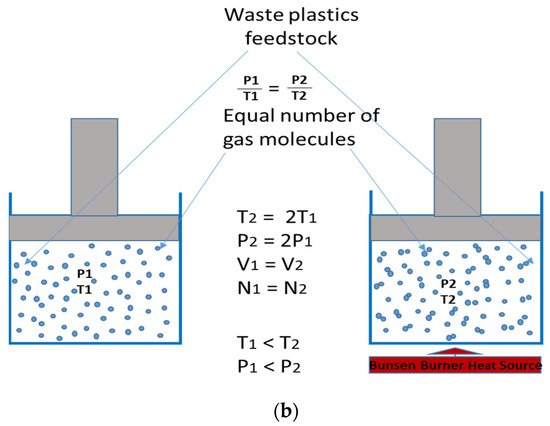
Figure 21b and supported by Gao and Sato and Sakata
[21][34].
Figure 21b gives a schematic glance into what Gay Lussac’s Law is, with waste plastics as feedstock materials. The reaction chamber with the heat source (T
2 < T
1) shows an increased motion of the gas molecules of the feedstock, hitting one another, thereby giving rise to a more pressurised system, and, hence, a gaseous yield.
With reference to Gao
[21], it is revealed that at high pressure, the yield of non-condensable gases increases, but with a decrease in the liquid yield. Lower molecular weight gases are typical examples of such non-condensable gases, and they include gases that constitute CO and CO
2. Partial vacuum, otherwise termed negative pressure, is a key factor in plastic pyrolysis as it ensures the minimisation of oxidation reactions, and further hastens the removal of gaseous vapours pitching towards the reduction of secondary reactions’ incidence in the process chamber of a pyrolytic reactor
[37]. Oxidation is bound to occur during plastic pyrolysis or even during oxidative pyrolysis, due to the presence of oxygen in the structure of plastics. Generally, nitrogen (N
2) can be used as the purging substance for the removal of air and related contaminants in the chamber, thus it does not necessarily participate in the reaction process. This is essential since pyrolysis constitutes oxidation; oxygen, water, or related reagents are barred. Furthermore, the elimination or the formulation of a barrier towards undesirable by-products is realised. As a result, during this process, the waste plastic is gently ‘cracked’ at relatively low temperatures to enhance the primarily straight-chain aliphatic HCs with a minimal formation of by-products. Thermal pressure is known to be a significant player, especially in the context of plastic recycling. According to a Central Pollution Control Board report, it takes between 2–3 times only as per virgin plastic material recycling, since plastic materials deteriorate due to the thermal pressure on every recycling process
[38].
However, high temperatures and heating rates, low pressures and residence times facilitate the formation of major products. Equally, long residence times lead to a prevalence of other steady products, such as methane and cooking gases
[10].
2. Active Commercial Plastic Pyrolysis Processes and Technologies
As many solutions are emergent on the management and useful handling of waste plastics and related polymeric wastes around the globe, many parts of the world have been and are continually developing pyrolysis mechanisms and other chemical reaction systems to enhance this. Thus, waste plastic pyrolysis (WPP) plants have been developed and built in many countries. In research conducted by Fivga et al. [39], an industrial-scale pyrolysis plant was modelled for production yield optimisation and economic viability of waste plastics into heavy fuel-oil alternatives. The net present value (NPV) and payout period (PO) of the plant were calculated technically to attain this. The calculated model is in support of the literature reported by Liu et al. [40] and Gao [41]. Aboulkas et al. [42] developed and utilised a special laboratory fluidised bed pyrolysis reactor to produce gasoline and styrene monomers with PE as feedstock. However, two key factors concerning the effectiveness and use of pyrolysis plants are tied to the effects of the feedstock composition and the adjoining technology. In Section 1.1, a detailed investigation is carried out on the chemical composition of the feedstock. The remainder of the subsections of Section 1 can be grouped under ‘technology’, as the second of two factors surrounding the effectiveness and utilisation of pyrolysis processes or pyrolysis plants. Thus, feedstock and the technology utilised are two of the most significant factors encompassing the effectiveness and utilisation of pyrolysis processes/plants.
3. A Brief Quality Guarantee Analysis of Waste Plastic Pyrolytic Liquid Fuel and Conventional Diesel
With reference to the New Zealand diesel regulations, 18 requirements are utilised in the conventional diesel comparison with plastic-derived fuels. These 18 requirements are grouped into thermodynamic properties, component distribution, performance properties and flow properties. Benzene, toluene and xylene (BTX) aromatics can be grouped under plastic-derived fuels. Jung et al.
[43] produced BTX aromatics from pyrolignic waste PE and PP plastics with the support of a fluidised bed reactor. The investigation centred on feed rate, decomposition temperature, and the influence of the fluidised bed on the product range. These properties align with two (thermodynamic properties and component distribution) of the four groups of the '18 requirements' for diesel comparison with plastic-derived fuels, as stated earlier. These same two groups can be linked to the HC yields of mixed plastics as reported by Sophonrat et al.
[44]. Distinct treatments are utilised in relation to the two product distributions of HCs and oxygenated materials obtained. The third group, performance properties can be connected to their study as per the circular economic benefits (useful industrial materials, chemicals and/or energy recoveries) of the products. The two products including the plastic-derived (cetane-index-based) product of HCs are produced via multi-step pyrolysis with reference to their various reaction temperatures that encompass the thermodynamic property group as explained above. From the regulation for commercial types of diesel, the cetane number or cetane index is the most significant thermodynamic properties, as they depict the auto-ignition conditions of the fuel
[45]. Fuel density and the distillation range are used for the cetane number calculation. These parameters can be found as part of the regulatory requirements; hence, all three properties (cetane index, fuel flow and fuel performance) are essential for the property makeup of diesel.
Essentially, the constituent elemental compositions for each of the waste plastics in comparison to either gasoline or diesel are approximately the same, but with a minimal difference in the sulphur (S) content, which can be taken care of in the pyrolysis of plastics. This highlights the fact that waste plastics can indeed be pyrolysed into liquid fuels or petrochemical products. It also proves that waste plastics are products of crude oil or petroleum. The high heating values (HHV) for PE is nearly equivalent to that of gasoline and diesel in the size of 46 MJ/kg
[43][46]. Going by this, PE being a significant feedstock in high yields of pyrolytic liquid fuels, as explained above, concludes the liquid-fuel potential associated with PEs, and is supported by a range of experimental kinds of literature.
4. Practical Implications of this Literature
The underlying practicalities of this research cover a range of benefits in the chemistry and chemical engineering fields, the environment, industrial-scale production and research supports, among other related implications. It reveals some common gaps and misconceptions surrounding waste plastics pyrolysis and provides clear arguments against them. These misconceptions and gaps such as pyrolysis not being a sustainable solution to waste plastics and the idea that waste plastic is indeed wealth due to its potential of being converted into fuel and other useful materials. Practically, these are challenges (current and/or future) embedded in this field. This literature offers a detailed understanding of the major factors that influence WPTP. Furthermore, the review paper aids the future work of other researchers in this field and will enable them to attain meaningful conclusions.