Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Hamza Mechchate.
The coronavirus disease 2019 (COVID-19) is an epidemic caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). Populations at risk as well as those who can develop serious complications are people with chronic diseases such as diabetes, hypertension, and the elderly. Severe symptoms of SARS-CoV-2 infection are associated with immune failure and dysfunction. The approach of strengthening immunity may be the right choice in order to save lives.
- SARS-CoV-2
- COVID-19
- immunity
- zinc
- polyphenols
- bee products
1. Introduction
On 11 March 2020, the World Health Organization recognized the new coronavirus disease 2019 (COVID-19) as a pandemic. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected for the first time in Wuhan, China, at the end of 2019; the virus has not reported before in humans [1]. The virus is highly contagious and is transmitted through close contact with the contaminated person via respiratory droplets from coughing, and/or direct contact with contaminated surfaces [2]. Security measures imposed include washing hands frequently with soap and water, disinfecting them with a hydroalcoholic solution, wearing a mask when leaving the house, keeping to at least one meter away from anyone, avoid touching eyes, nose, or mouth when coughing or sneezing, and covering the nose and mouth with the bend of the elbow or with a tissue [3]. Most infected people have mild to moderate disease symptoms. The most frequent symptoms are fever, dry cough, fatigue, in addition to body aches, sore throat, diarrhea, conjunctivitis, headache, loss of smell or taste, rash, or discoloration of the fingers or toes; the most serious symptoms include difficulty in breathing or shortness of breath, feeling of tightness or pain in the chest, and loss of speech or motor skills [4,5][4][5]. Some infected people may be asymptomatic but they transmit the virus to other persons. Currently, there is no specific treatment for coronavirus infection. In non-hospitalized people, treatment aims to relieve symptoms and the most recommended active ingredient is paracetamol [6]. In other patients, doctors have introduced other drugs to treat the disease; these drugs were originally developed to treat other pathologies such as immunomodulatory drugs (e.g., interferons), and antiviral drugs which act by inhibiting the entry of the virus inside the cell (e.g., hydroxychloroquine, niclosamide, camostat), or by inhibiting viral proteases (e.g., lopinavir/ritonavir, PF-07321332, GC376, PF-07304814), other drugs inhibit viral RNA (e.g., remdesivir, molnupiravir), and finally the drugs that inhibit the host proteins which support viral protein synthesis (e.g., plitidepsin, fluvoxamine) [7]. Recently, Pfizer (New York, United States) has announced a new oral antiviral drug, ritonavir in combination with PF-07321332 (PAXLOVID™), that significantly reduces hospitalization and death by 89% of COVID-19 patients at high risk of severe disease [8,9][8][9]. The problem that still arises is the safety profile of these drugs. They require further evaluation such as evaluating the possible drug-drug interactions [10]. In addition, certain drugs are expensive and require intravenous administration supervised in a hospital [11].
The reason for the growing consumption of beehive products may be related to their health-oriented and therapeutic properties. The lookout for published studies on bee products and their ability to interact with SARS-CoV-2 and alleviate the symptoms of COVID-19 has attracted a lot of attention as they present a promising source of natural substances that can reduce severe symptoms of infected patients [12,13][12][13]. It is worth mentioning that several clinical trials have tested the combination between bee products and standard care to treat COVID-19 patients. For instance, in the trial number NCT04323345, there was a combination of honey given orally and through a nasogastric tube and standard drugs (lopinavir/ritonavir tablets or arbidol or chloroquine phosphate or hydroxychloroquine or oseltamivir with or without azithromycin) [14]. In addition, extract of standardized Brazilian green propolis, EPP-AF® combined with standard care (azithromycin, chloroquine or hydroxychloroquine, oseltamivir, corticosteroids) were used for hospitalized COVID-19 patients in a trial registered under the number NCT04480593 [15]. The outcomes of these studies revealed that combining these natural products with standard care procedures has resulted in clinical benefits for hospitalized COVID-19 patients in comparison to the patients who only received standard care, such as the reduction in hospital stay length.
2. Mechanisms of the Immune Response against SARS-CoV-2
The immune system refers to body’s global ability to resist and defend against different infections (fungi, protozoan, bacteria, and viruses). SARS-CoV-2 is one of these new pathogenic agents that triggers a coordinated immune response: innate immunity (rapid response), adaptive immunity (lower-acting), and passive immunity. When the human body encounters pathogenic antigens for the first time, in some people, especially vulnerable individuals the immune system cannot function quickly, complications may arise [16]. This has been observed in the case of COVID-19. The interface between innate and adaptive immunity immediately begins when the virus (SARS-CoV-2) reaches host cells [17]. This process goes through three essential steps: the identification of the spike glycoprotein of the virus, the elimination of the virus and the infected cells, and finally the development of immunological memory.
2.1.1. The Identification of the Spike Glycoprotein of SARS-CoV-2
When the functional barrier of the immune system (respiratory tract and acid pH of the stomach etc.) fails to block SARS-CoV-2, monocytes, macrophages, and dendritic cells, antigen-presenting cells (APC) recognize and achieve it through the presence of pathogen recognition receptors (PRRs). Then, the first line of host defensive responses is activated. Owing to their intracellular Toll-like receptors (TLRs) especially, TLR3, TLR7, and TLR8, monocytes, macrophages, dendritic cells, and some other cell types recognize the single-stranded RNA of coronaviruses including SARS-CoV-2. Besides, extracellular PRRs identify the spike glycoprotein of the coronavirus coat (Figure 1A) [18,19][18][19].
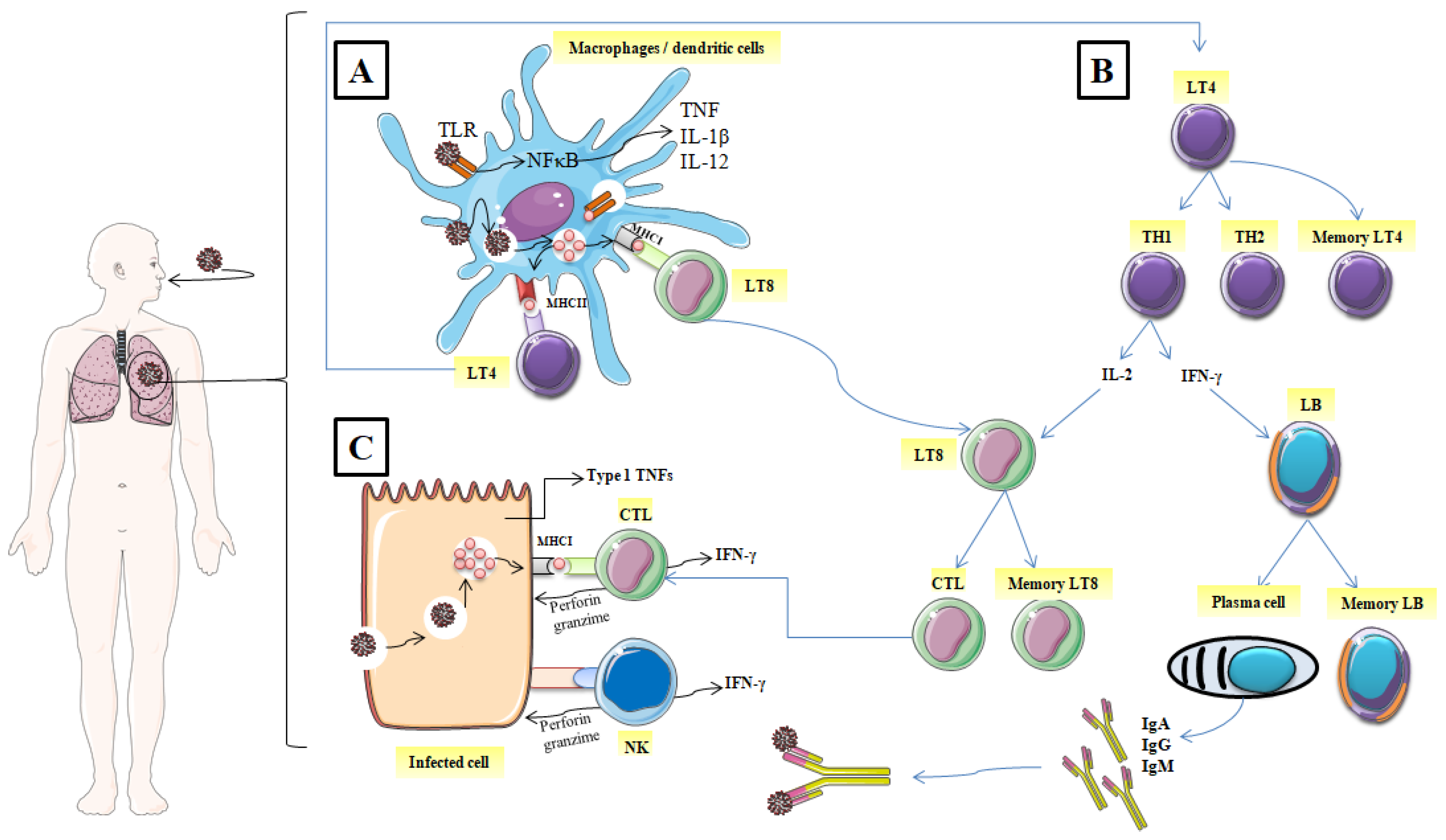
Figure 1. Mechanisms of the immune response against SARS-CoV-2. (A) identification of the spike glycoprotein of SARS-CoV-2, (B) activation of CD4+ helper T lymphocytes, (C) Elimination of the virus and the infected cell. (TLR: Toll-like receptors; NF-κB: nuclear factor-kappa B; TNF: tumor necrosis factor; IL: interleukin; MHCI: major histocompatibility class I; MCHII: major histocompatibility class I; LT4: CD4+ helper T lymphocyte; LT8: CD8+ helper T lymphocytes; TH1: T helper 1; TH2: T helper 2; IFNγ: interferon, LB: lymphocyte B; CTL: cytotoxic T lymphocyte; NK: natural killer; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M).
2.1.2. Elimination of the Virus, and the Infected Cell
Virally infected cells present on their surface virus antigenic determinants by major histocompatibility class I (MHC I), which are subsequently recognized by antigen-specific CD8+ cytotoxic T lymphocytes and then induce their restriction by the release of the effectors’ molecules such as perforin and granzymes (Figure 1C) [19]. Likewise, Natural killer cells (NK) also recognize and kill the infected host cells. The host immune cells adopted this strategy to slow down the virus invasion and thus, the interaction between the immune system and virally infected cells continues.
After the digestion of the internalized virus, termed antigens are recognized by specific TLRs and present on the surface of the innate immune cells (macrophage or dendritic cells) via MHC I (MHC I); [Human Leukocyte Antigen (HLA) in humans] and MHC II (HLA-Cw∗08) [20]. Furthermore, the recognition of antigen by PRRs enhances the expression of typical inflammatory cytokines such as IL-2, IL-18, IL-1β, type 1 IFNs (IFN-α and IFN-β), and tumor necrosis factor (TNF); it also activates inflammatory signaling and transcription factors such as inflammasome assembly and nuclear factor kappa-B cells (NFκB) [21]. In turn, these inflammatory cytokines initiate the activation of CD4+ helper T lymphocytes with the transition into a T helper (Th) which has a double function: cytokines produce and promote B Cells to generate antibodies. Being stimulated by intracellular pathogens, T helper lymphocytes 1 (Th1) phenotype promotes the cytotoxic T lymphocyte activity by releasing IL-2 and enhances the differentiation of B lymphocytes to plasma cells which produce specific antiviral antibodies by liberating IFN-γ (Figure 1B).
T helper lymphocytes (Th2) activate and promote the degranulation and the release of chemokines, proteases, and histamines by innate immune system cells including mast cells and basophils, which increased vascular permeability and enhanced the recruitment of macrophages and other inflammatory cells [22]. Besides, Th2 migrate to lung tissue and act by inducing airway hyper-responsiveness and metaplasia of the Goblet cells as documented in allergic illnesses [23]. Interactions (between TH1, TH2, and APC) are generally described as cellular cooperation.
2.1.3. Immunological Memory
Thanks to immunological memory, the immune system can specifically recognize and immediately trigger an adequate immune response upon re-contact with an antigen previously encountered by the body (Figure 1B). In addition to the specific antibodies released into the circulation (IgM, IgA, and IgG), after the end of the active immune response, a pool of T memory cells are ready to fight against re-infection, leading to a fast elimination of the specific antigen source [24]. It has been observed that both CD8+ and CD4+ memory T cells in SARS-CoV-2 patients were effective in prompting a specific immune response from 3 months to 6 years without the presence of antigens [25]. Specific humoral immune response (anti-S-RBD IgM and anti-N IgG) against SARS-CoV-2 was found to have similar characteristics to that triggered against other coronavirus infections. IgG antibodies appear within 14 days of the onset of initial symptoms. However, IgA and IgM were detected on the fifth day after the first symptoms [26]. Positivity rates for IgM, IgA and IgG reached their maximum at weeks 4, 5 and 6, respectively [27].
References
- Mütze, T.; Friede, T. Data Monitoring Committees for Clinical Trials Evaluating Treatments of COVID-19. Contemp. Clin. Trials 2020, 98, 106154.
- Ather, A.; Patel, B.; Ruparel, N.B.; Diogenes, A.; Hargreaves, K.M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J. Endod. 2020, 46, 584–595.
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020.
- Mullol, J.; Alobid, I.; Mariño-Sánchez, F.; Izquierdo-Domínguez, A.; Marin, C.; Klimek, L.; Wang, D.-Y.; Liu, Z. The Loss of Smell and Taste in the COVID-19 Outbreak: A Tale of Many Countries. Curr. Allergy Asthma Rep. 2020, 20, 61.
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The Emergence of COVID-19 as a Global Pandemic: Understanding the Epidemiology, Immune Response and Potential Therapeutic Targets of SARS-CoV-2. Biochimie 2020, 179, 85–100.
- Samaee, H.; Mohsenzadegan, M.; Ala, S.; Maroufi, S.S.; Moradimajd, P. Tocilizumab for Treatment Patients with COVID-19: Recommended Medication for Novel Disease. Int. Immunopharmacol. 2020, 89, 107018.
- Yavuz, S.; Çelikyurt, F.I.K. An Update of Anti-Viral Treatment of COVID-19. Turk. J. Med. Sci. 2021, 51, 3372–3390.
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An Update on Drugs with Therapeutic Potential for SARS-CoV-2 (COVID-19) Treatment. Drug Resist. Updat. 2021, 59, 100794.
- Bolarin, J.A.; Oluwatoyosi, M.A.; Orege, J.I.; Ayeni, E.A.; Ibrahim, Y.A.; Adeyemi, S.B.; Tiamiyu, B.B.; Gbadegesin, L.A.; Akinyemi, T.O.; Odoh, C.K. Therapeutic Drugs for SARS-CoV-2 Treatment: Current State and Perspective. Int. Immunopharmacol. 2021, 90, 107228.
- Heskin, J.; Pallett, S.J.; Mughal, N.; Davies, G.W.; Moore, L.S.; Rayment, M.; Jones, R. Caution Required with Use of Ritonavir-Boosted PF-07321332 in COVID-19 Management. Lancet 2022, 399, 21–22.
- Parums, D.V. Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935952.
- Lima, W.G.; Brito, J.C.; da Cruz Nizer, W.S. Bee Products as a Source of Promising Therapeutic and Chemoprophylaxis Strategies against COVID-19 (SARS-CoV-2). Phytother. Res. 2021, 35, 743–750.
- Elmahallawy, E.K.; Mohamed, Y.; Abdo, W.; El-Gohary, F.A.; Ahmed Awad Ali, S.; Yanai, T. New Insights into Potential Benefits of Bioactive Compounds of Bee Products on COVID-19: A Review and Assessment of Recent Research. Front. Mol. Biosci. 2021, 7, 513.
- Tantawy, M. Efficacy of Natural Honey Treatment in Patients with Novel Coronavirus. Clinicaltrials.Gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04323345 (accessed on 11 February 2022).
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.D.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; Conceição, L.F.M.R.D.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian Green Propolis (EPP-AF®) as an Adjunct Treatment for Hospitalized COVID-19 Patients: A Randomized, Controlled Clinical Trial. Biomed. Pharmacother. 2021, 138, 111526.
- Hoebe, K.; Janssen, E.; Beutler, B. The Interface between Innate and Adaptive Immunity. Nat. Immunol. 2004, 5, 971–974.
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune Response in COVID-19: A Review. J. Infect. Public Health 2020, 13, 1619–1629.
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS Coronavirus Spike Protein-Induced Innate Immune Response Occurs via Activation of the NF-KappaB Pathway in Human Monocyte Macrophages in Vitro. Virus Res. 2009, 142, 19–27.
- Hu, W.; Yen, Y.-T.; Singh, S.; Kao, C.-L.; Wu-Hsieh, B.A. SARS-CoV Regulates Immune Function-Related Gene Expression in Human Monocytic Cells. Viral Immunol. 2012, 25, 277–288.
- Chen, Y.-M.A.; Liang, S.-Y.; Shih, Y.-P.; Chen, C.-Y.; Lee, Y.-M.; Chang, L.; Jung, S.-Y.; Ho, M.-S.; Liang, K.-Y.; Chen, H.-Y.; et al. Epidemiological and Genetic Correlates of Severe Acute Respiratory Syndrome Coronavirus Infection in the Hospital with the Highest Nosocomial Infection Rate in Taiwan in 2003. J. Clin. Microbiol. 2006, 44, 359–365.
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological Decision-Making: How Does the Immune System Decide to Mount a Helper T-Cell Response? Immunology 2008, 123, 326–338.
- Anaya, J.-M.; Shoenfeld, Y.; Rojas-Villarraga, A.; Levy, R.A.; Cervera, R. Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013; ISBN 958-738-366-4.
- Paul, W.E.; Zhu, J. How Are T(H)2-Type Immune Responses Initiated and Amplified? Nat. Rev. Immunol. 2010, 10, 225–235.
- Stockinger, B.; Bourgeois, C.; Kassiotis, G. CD4+ Memory T Cells: Functional Differentiation and Homeostasis. Immunol. Rev. 2006, 211, 39–48.
- Fan, Y.-Y.; Huang, Z.-T.; Li, L.; Wu, M.-H.; Yu, T.; Koup, R.A.; Bailer, R.T.; Wu, C.-Y. Characterization of SARS-CoV-Specific Memory T Cells from Recovered Individuals 4 Years after Infection. Arch. Virol. 2009, 154, 1093–1099.
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 778–785.
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240.
More
