Brominated flame retardants (BFRs) are the compounds used to reduce flammability and/or retard combustion of organic materials, mostly of polymerized plastics. Despite increasing regulations and a ban on the use of selected BFRs, the compounds of this family, such as TBBPA are still widely used on the market. The widespread use of BFRs contributes to the exposure of the environmental and the human.
- brominated flame retardants
- tetrabromobisphenol A
- toxicity
- polybrominated diphenylethers
- hexabromocyclododecanes
1. Introduction
An unprecedented consumption growth of the flammable, synthetic polymer materials, such as polyvinyl chloride, in recent decades, have contributed to the development of flame retardants (FRs) market. In Europe, FRs containing bromine account for about 25% of all FRs, while in China it is approximately 40%.
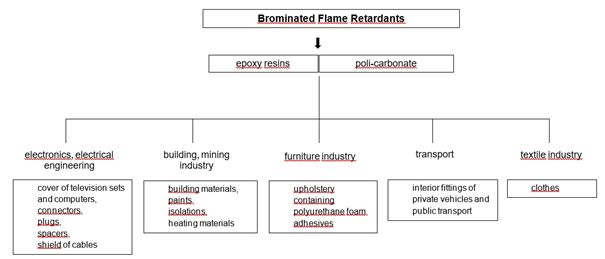
BFRs are commonly used to prevent fires in electronic and electrical equipment, which accounts for more than 50% of their applications. In addition, BFRs are used to reduce the flammability of plastics in many products, such as household articles, furniture, mattresses, textiles or insulation (scheme 1).
Scheme 1. Application of BFRs [1].
Among BFRs there are over 80 different chemical substances, with two groups being distinguished, i.e. additive compounds, which include polybrominated diphenylethers (PBDEs) or hexabromocyclododecanes (HBCDs) and reactive compounds, which include e.g. tetrabromobisphenol A (TBBPA). The first are mixed with the remaining components of the polymer material during, before or after the polyreaction, which promotes their release into the environment. On the other hand, reactive compounds can be incorporated into the polymer chain during the polymerization reaction, which hinders the possibility of migration from the product to the environment.
2. PBDEs and HCBDs
Until recently, PBDEs were the most widely used compounds among BFRs. High production and usage in the past years, as well as their hydrophobicity and persistence in the environment have contributed to a significant contamination of the environment including soil [2][3][4], air [5][6], water [7] and sediment samples [8] as well as humans [9].
Hexabromocyclododecanes (HBCDs) are the most common BFRs after PBDEs. Environmental contamination with HBCDs has been detected globally, with concentrations ranging from ng to μg. HBCDs have been detected as pollutants in various environments and biological samples, including human[10].
PBDEs and HBCDs have been recognized as global pollutants associated with numerous adverse health effects including infertility, hormone metabolism, neurotoxicity and neoplastic processes and, therefore, withdrawn from production in the European Union [11][12].
Despite increasing regulations and a ban on the use of selected BFRs, the compounds of this family are still widely used on the market.
3. Tetrabromobisphenol A (TBBPA)
TBBPA is the most commonly used BFR with production of about 60% of all BFRs [13]. The withdrawal of PBDEs and HBCDs from the market has contributed to the increase in TBBPA demand. The annual production of this chemical has been estimated at around 220,000 tones [14]. Despite such a high demand and usage of this compound, and due to the fact that it is present in the environment in lower concentrations than other BFRs such as PBDEs and HBCDs, as well as the fact that over 80% of it is used as a reactive compound, which limits the possibility of its migration from product to the environmental. so far there are no restrictions concerning to TBBPA [15].
The widespread use of TBBPA contributes to the exposure of the environmental and the human. Numerous studies have documented the presence of TBBPA in the environment. TBBPA was determined in soil, water, air [16][17][18][19]. TBBPA, due to high concentrations in the environment and human surrounding, may constitute a real hazard. Due to the high hydrophobicity of this compound, it can be accumulated in the nutritive chain in living organisms, including humans. TBBPA has been identified in human biological materials such as plasma, maternal milk or adipose tissue [20][21][22].
Few studies have reported the potential toxicity of high doses of TBBPA for mammals. Choi et al. (2011) have demonstrated that, as a consequence of repeated exposure of rats to high TBBPA doses, a significant decrease in serum thyroxine level, liver weight and cytochrome CYP2B1 induction was observed. In addition, TBBPA has been shown to induce reactive oxygen species (RFT), thereby enhancing toxicity to the liver, kidneys and testes [23]. Moreover, it is supposed that TBBPA contributes to the development of variety disorders like cancer, diabetes or obesity [24][25][26][27]. Furthermore, TBBPA at very low concentrations caused an increase of reactive oxygen species (ROS) production [28], altered the activity of the antioxidative system [29] as well as inducing suicidal cell death (eryptosis) [30] and hemolysis in erythrocytes (red blood cells, RBCs) [28]. Moreover, TBBPA caused oxidative changes in erythrocytes membrane proteins, and of the model HSA protein and changed albumin conformation properties. Potential binding of BFRs to proteins or their indirect action on protein molecules, e.g., by ROS production, may cause structural and functional changes in proteins, and this may explain some of the pathological conditions observed in chronic BFR exposure [31].
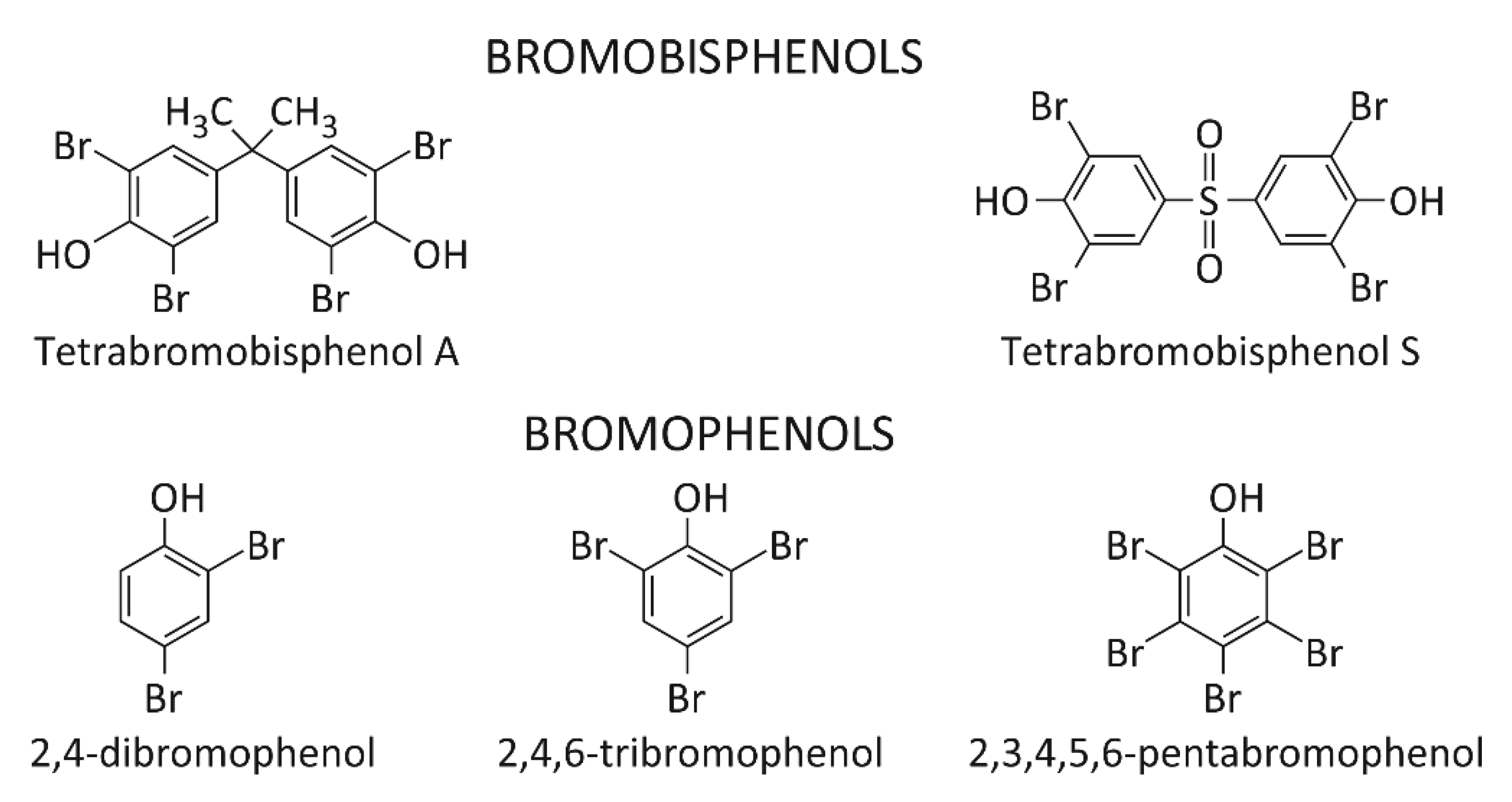
Due to the reports concerning toxicity of TBBPA, tetrabromobisphenol S (TBBPS) has been introduced into the market as substitute of this compound.
4. Other BFRs
There are only a few studies referring to environmental exposure and toxicity of other BFRs such as bromophenols (scheme 2). 2,4-dibromophenol (2,4-DBP) and 2,4,6-tribromophenol (2,4,6-TBP) may be present in the environment not only as a result of human activity (used as a intermediate in the production of flame retardants). 2,4,6-TBP is produced by marine organisms to defend against predators. It was estimated that in 2001 the demand for 2,4,6-TBP reached the value of 9100 tons [32]. 2,4,6-TBP was determined in maternal and umbilical blood [33]. This compound was also detected in breast milk, urine and placental tissue [34][35][36][37]. Pentabromophenol (PBP) is used as a flame retardant and is the main biological metabolite of PBDEs. It has been found that it can modulate TGF-ß signaling. TGF-β is a major factor in the regulation of a wide variety of biological processes including cell proliferation, migration, and development of cancer [38]. Moreover, it was found that the structure of BFRs differing in the number of aromatic rings and the number of bromine atoms in the ring significantly affected their interaction with proteins [31].
References
- Jarosiewicz, M., Bukowska, B., 2017. Tetrabromobisfenol A–toksyczność, narażenie środowiskowe i zawodowe. Med. Pr. 68, 121-134.
- Eljarrat, E., Marsh, G., Labandeira, A., and Barceló, D. 2008. Effect of sewage sludge contaminated with polybrominated diphenylethers on agricultural soils. Chemosphere, 71(6): 1079–1086. doi:10.1016/j.chemosphere.2007.10.047.
- Tang, X., Zeng, B., Hashmi, M.Z., Long, D., Yu, B., Ullah, N., Shen, C., and Chen, Y. 2014. PBDEs and PCDD/Fs in surface soil taken from the Taizhou e-waste recycling area, China. Chem. Ecol. 30(3): 245-251. doi:10.1080/02757540.2013.844798.
- Wu, M.H., Pei, J.C., Zheng, M., Tang, L., Bao, Y.Y., Xu, B.T., et al. 2015. Polybrominated diphenyl ethers (PBDEs) in soil and outdoor dust from a multi-functional area of Shanghai: Levels, compositional profiles and interrelationships. Chemosphere 118: 87-95. doi:10.1016/j.chemosphere.2014.06.022
- Moeller, A., Xie, Z., Sturm, R., Ebinghaus, R. 2011. Polybrominated diphenyl ethers (PBDEs) and alternative brominated flame retardants in air and seawater of the European Arctic. Environ. Pollut. 159: 1577-1583.
- Bennet, D.H., Moran, R.E., Wu, X., Tulve, N.S., Clifton, M.S., Colon, M., Weathers, W., Sjodin, A., Jones, R., Hertz-Picciotto, I. 2015. Polybrominated diphenyl ether (PBDE) concentrations and resulting exposure in homes in California: relationships among passive air, surface wipe and dust concentrations, and temporal variability. Indoor Air 25: 220-229. doi:10.1111/ina.12130.
- Liu, Y., Zheng, G.J., Yu, H.X., Martin, M., Richardson, B.J., Lam, M.H.W., Lam, P.K.S., 2005. Polybrominated diphenyl ethers (PBDEs) in sediments and mussel tissues from Hong Kong marine waters. Marine Pollution Bulletin 50: 1173-1184.
- Li, Q., Yan, C., Luo, Z., and Zhang, X., 2010. Occurrence and levels of polybrominated diphenyl ethers (PBDEs) in recent sediments and marine organisms from Xiamen offshore areas, China. Marine Pollution Bulletin 60: 464-469.
- Dimitriadou, L., Malarvannan, G., Covaci, A., Iossifidou, E., Tzafettas, J., et. al., 2016. Levels and profiles of brominated and chlorinated contaminants in human breast milk from Thessaloniki, Greece. Sci. of the Total Environ.539, 350-358. doi:10.1016/j.scitotenv.2015.08.137.
- Huang, L., Shah, S. B., Hu, H., Xu, P., Tang, H. 2020. Pollution and biodegradation of hexabromocyclododecanes: A review. Frontiers of Environmental Science & Engineering, 14(1),11.
- Eljarrat E., Barcelo D., [red.], 2011. Brominated flame retardants. Springer Berlin Heidelberg, Berlin, https://doi.org/10.1007/978-3-642-19269-2.
- Shaw, S. D., Harris, J. H., Berger, M. L., Subedi, B., Kannan, K., 2014. Brominated flame retardants and their replacements in food packaging and household products: Uses, human exposure, and health effects. In Toxicants in Food Packaging and Household Plastics (pp. 61-93). Springer, London.
- Law, R. J., Bersuder, P., Allchin, C. R., Barry, J., 2006. Levels of the flame retardants hexabromocyclododecane and tetrabromobisphenol A in the blubber of harbor porpoises (Phocoena phocoena) stranded or bycaught in the UK, with evidence for an increase in HBCD concentrations in recent years. Environmental science & technology, 40(7), 2177-2183.
- Covaci, A., Voorspoels, S., Abdallah, M. A. E., Geens, T., Harrad, S., Law, R. J., 2009. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J. Chromatogr. A 1216, 346-363.
- Lai, D. Y., Kacew, S., Dekant, W., 2015. Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents. Food Chem. Toxicol., 80, 206-2014.
- Tang, J., Feng, J., Li, X., Li, G., 2014. Levels of flame retardants HBCD, TBBPA and TBC in surface soils from an industrialized region of East China. Environ. Sci. Process. Impacts 16, 1015-1021. https://doi.org/10.1039/ c3em00656e.
- Gorga, M., Martínez, E., Ginebreda, A., Eljarrat, E., Barceló, D., 2013. Determination of PBDEs, HBB, PBEB, DBDPE, HBCD, TBBPA and related compounds in sewage sludge from Catalonia (Spain). Sci. Total Environ., 444, 51-59.
- Kowalski, B., Mazur, M., 2014. The simultaneous determination of six flame retardants in water samples using SPE preconcentration and UHPLC-UV method. Water Air Soil Pollut. 225(1886):1–9.
- Xie, Z., Ebinghaus, R., Lohmann, R., Heemken, O., Caba, A., Püttmann, W., 2007. Trace determination of the flame retardant tetrabromobisphenol A in the atmosphere by gas chromatography–mass spectrometry. Anal. Chim. Acta 584, 333-342, https://doi.org/10.1016/j.aca.2006. 10.062.
- Kim, U.J., Oh, J.E., 2014. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ. Pollut., 184:193–200.
- Shi, Z., Jiao, Y., Hu, Y., Sun, Z., Zhou, X., Feng, J. et al., 2013. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ., 452(453):10–18.
- Johnson-Restrepo, B., Adams, D.H., Kannan, K., 2008. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and shark from the United States. Chemosphere, 70(11):1935–1944.
- Choi, J.S., Lee, Y.J., Kim, T.H., Lim, H.J., Ahn, M.Y., Kwack, S.J., Kang, T.S., Park, K.L., Lee, J., Kim, N.D., Jeong, T.C., Kim, S.G., Jeong, H.G., Lee, B.M., Kim, H.S., 2011. Molecular mechanism of tetrabromobisphenol A (TBBPA)-induced target organ toxicity in Sprague-Dawley male rats. Toxicol. Res. 27(2), 61-70.
- Dunnick, J. K., Sanders, J. M., Kissling, G. E., Johnson, C. L., Boyle, M. H., Elmore S. A., 2015. Environmental chemical exposure may contribute to uterine cancer development: studies with tetrabromobisphenol A. Toxicol. Pathol. 43(4), 464-473.
- Hall, S. M., Knudsen, G. A., Coulter, S. J., Sanders, J. M., Birnbaum, L. S., 2015. Changes in gene expression and pathway effects of tetrabromobisphenol A (TBBPA) in female Wistar Han rats. Cell Mol. Biol., 7, 180–182.
- McCollum, CW, Riu A, Bondesson, M, Gustafsson, JA. The Endocrine Society's 94th Annual Meeting and Expo, June 23–26.2012 - Houston, TX. Obesity: An Effect of Environmental Pollutants? Date of Presentation: June 24.2012. Presentation number: SUN-136.
- Barret, J.: Warm Reception? Halogenated BPA Flame Retardants and PPARγ Activation. Environ Health Perspect. 2011;119(9): a398.
- Jarosiewicz, M., Duchnowicz, P., Włuka, A., Bukowska, B., 2017. Evaluation of the effect of brominated flame retardants on hemoglobin oxidation and hemolysis in human erythrocytes. Food Chem. Toxicol. 109, 264-271.
- Jarosiewicz, M., Krokosz, A., Marczak, A., Bukowska, B., 2019. Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere, 227, 93-99.
- Jarosiewicz, M., Michałowicz, J., Bukowska, B., 2019. In vitro assessment of eryptotic potential of tetrabromobisphenol A and other bromophenolic flame retardants. Chemosphere, 215, 404-412.
- Jarosiewicz, M., Miłowska, K., Krokosz, A., Bukowska, B., 2020. Evaluation of the Effect of Selected Brominated Flame Retardants on Human Serum Albumin and Human Erythrocyte Membrane Proteins. Int. J. Molec. Sci., 21(11), 3926.
- Koch, C., Sures, B., 2018. Environmental concentrations and toxicology of 2, 4, 6-tribromophenol (TBP). Environ. Pollut. 233, 706-713.
- Kawashiro, Y., Fukata, H., Omori-Inoue, M., Kubonoya, K., Jotaki, T., Takigam, H., Sakai, S., Mori, C., 2008. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocr. J. 55, 1071-1084.
- Ohta, S., Okumura, T., Nishimura, H., Nakao, T., Shimizu, Y., Ochiai, F., Aozasa, O., Miyata, H., 2004. Human level of PBDEs, TBBPA, TBPs, PCDDs/DFs, PXDDs/DFs and PBDDs/DFs in human milk of nursing woman and dairy milk products in Japan. Organohalogen Compd. 66, 2857-2862.
- Feng, C., Xu, Q., Lin, Y., Qiu, X., Lu, D., Wang, G., 2016. Determination of urinary bromophenols (BrPs) as potential biomarkers for human exposure to polybrominated diphenyl ethers (PBDEs) using gas chromatography-tandem mass spectrometry (GC–MS/MS). J. Chromatogr. B 1022, 70-74.
- Nichkova, M., Marco, M. P., 2006. Biomonitoring human exposure to organohalogenated substances by measuring urinary chlorophenols using a high-throughput screening (HTS) immunochemical method. Environ. Sci. Technol. 40, 2469-2477.
- Smeds, A., Saukko, P., 2003. Brominated flame retardants and phenolic endocrine disrupters in Finnish human adipose tissue. Chemosphere 53, 1123-1130.
- Chen, C. L., Yang, P. H., Kao, Y. C., Chen, P. Y., Chung, C. L., Wang, S. W., 2017. Pentabromophenol suppresses TGF-β signaling by accelerating degradation of type II TGF-β receptors via caveolae-mediated endocytosis. Sci. Rep. 7, 43206. doi: 10.1038/ srep43206.