Deoxynivalenol is a toxic compound produced by filamentous fungi and represents a threat to public health. It is not possible to totally extinguish fungal contamination in crops such as wheat and corn and thereby avoid the production of this toxin. Its occurrence, regulation, ingestion, metabolism, and toxicology to animals, especially swine and poultry are discussed in this topic review.
- Deoxynivalenol
- Phylogeny
- DON
- Mycotoxins
1. Introduction
2. Occurrence, Regulation, Ingestion, and Metabolism
Agency | Specifications | Limit (mg/kg) | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
FDA (United States) | Grains and grain by-products destined for ruminating beef and feedlot cattle older than 4 months and for chicken | 10 | [14] | ||||||||
Grain and grain by-products destined for swine and other animals | 5 | ||||||||||
EFSA (EU) | Cereals and cereal products except for maize by-products | 8 | [17] | ||||||||
Maize by-products | 12 | ||||||||||
Complementary and complete feeding stuffs for animals | 5 | ||||||||||
Complementary and complete feeding stuffs for pigs | 0.9 | ||||||||||
Food Inspection Agency (Canada) | Diets for cattle and poultry | 5 | [15] | ||||||||
Diets for swine, young calves, and lactating dairy animals | 1 | ||||||||||
Department of Agriculture, Forestry, and Fisheries (South Africa) | Feeding stuffs on a full ration basis for: | [16] | |||||||||
Pigs | 1 | ||||||||||
Cattle | 5 | ||||||||||
Calves up to 4 months | 2 | ||||||||||
Dairy Cattle | 3 | ||||||||||
Poultry | 4 |
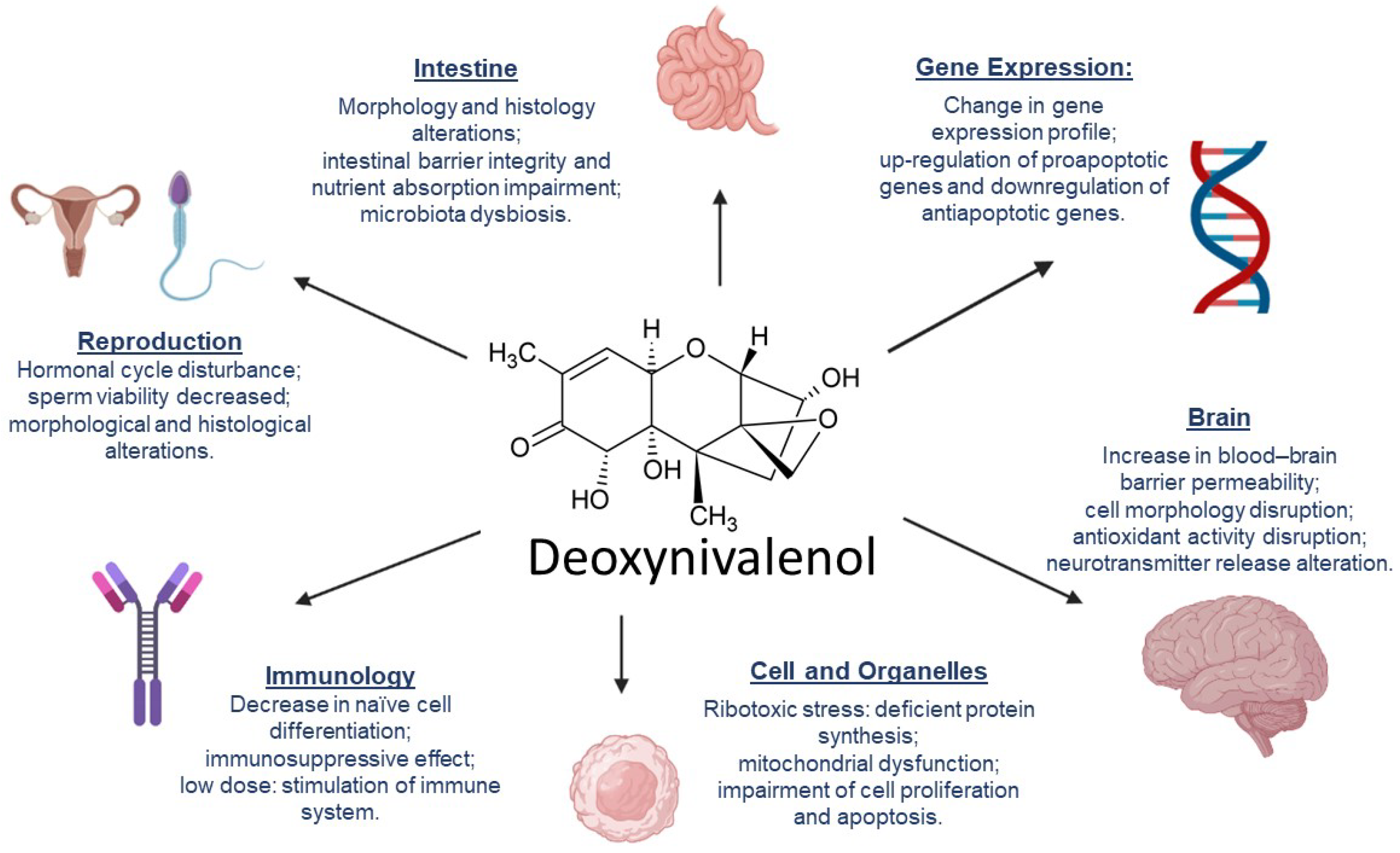
3. DON’s Mechanisms of Toxicity
References
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171.
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; IntechOpen: London, UK, 2020.
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146.
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789.
- Cope, R.B. Trichothecenes. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1043–1053.
- Pallez-Barthel, M.; Cocco, E.; Vogelgsang, S.; Beyer, M. Frequency of Deoxynivalenol Concentrations above the Maximum Limit in Raw Winter Wheat Grain during a 12-Year Multi-Site Survey. Agronomy 2021, 11, 960.
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300.
- Ramirez, M.L.; Chulze, S.; Magan, N. Temperature and water activity effects on growth and temporal deoxynivalenol production by two Argentinean strains of Fusarium graminearum on irradiated wheat grain. Int. J. Food Microbiol. 2006, 106, 291–296.
- Beyer, M.; Klix, M.B.; Klink, H.; Verreet, J.-A. Quantifying the effects of previous crop, tillage, cultivar and triazole fungicides on the deoxynivalenol content of wheat grain—A review. J. Plant Dis. Prot. 2006, 113, 241–246.
- Biomin Pesquisa Mundial de Micotoxinas: Impacto em 2021. Available online: https://www.biomin.net/br/science-hub/pesquisa-mundial-de-micotoxinas-impacto-em-2021/ (accessed on 30 September 2021).
- Herrera, M.; Bervis, N.; Carramiñana, J.J.; Juan, T.; Herrera, A.; Ariño, A.; Lorán, S. Occurrence and Exposure Assessment of Aflatoxins and Deoxynivalenol in Cereal-Based Baby Foods for Infants. Toxins 2019, 11, 150.
- FAO Worldwide Regulations for Mycotoxins in Food and Feed in 2003. Available online: http://www.fao.org/3/y5499e/y5499e00.htm (accessed on 27 September 2021).
- Romer Labs Worldwide Mycotoxin Regulations. Available online: https://www.romerlabs.com/en/knowledge-center/knowledge-library/articles/news/worldwide-mycotoxin-regulations/ (accessed on 27 September 2021).
- FDA Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human (accessed on 6 October 2021).
- Canadian Food Inspection Agency RG-8 Regulatory Guidance: Contaminants in Feed. Available online: https://inspection.canada.ca/animal-health/livestock-feeds/regulatory-guidance/rg-8/eng/1347383943203/1347384015909?chap=1 (accessed on 2 October 2021).
- Department of Agriculture, Forestry and Fisheries of South Africa. Farm Feeds Regulations: Amendment Government Notices No. R. 70 of 12 February 2010. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/3293570.pdf (accessed on 2 October 2021).
- Ec Recomendação Da Comissão 2006/576 de 17 de Agosto de 2006 Sobre a Presença de Desoxinivalenol, Zearalenona, Ocratoxina A, Toxinas T-2 e HT-2 e Fumonisinas em Produtos Destinados à Alimentação Animal. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0576 (accessed on 7 October 2021).
- DAF Mould, Poisons and Other Toxins. Available online: https://www.daf.qld.gov.au/business-priorities/agriculture/animals/pigs/health-diseases/mould-poisons-toxins (accessed on 6 October 2021).
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108.
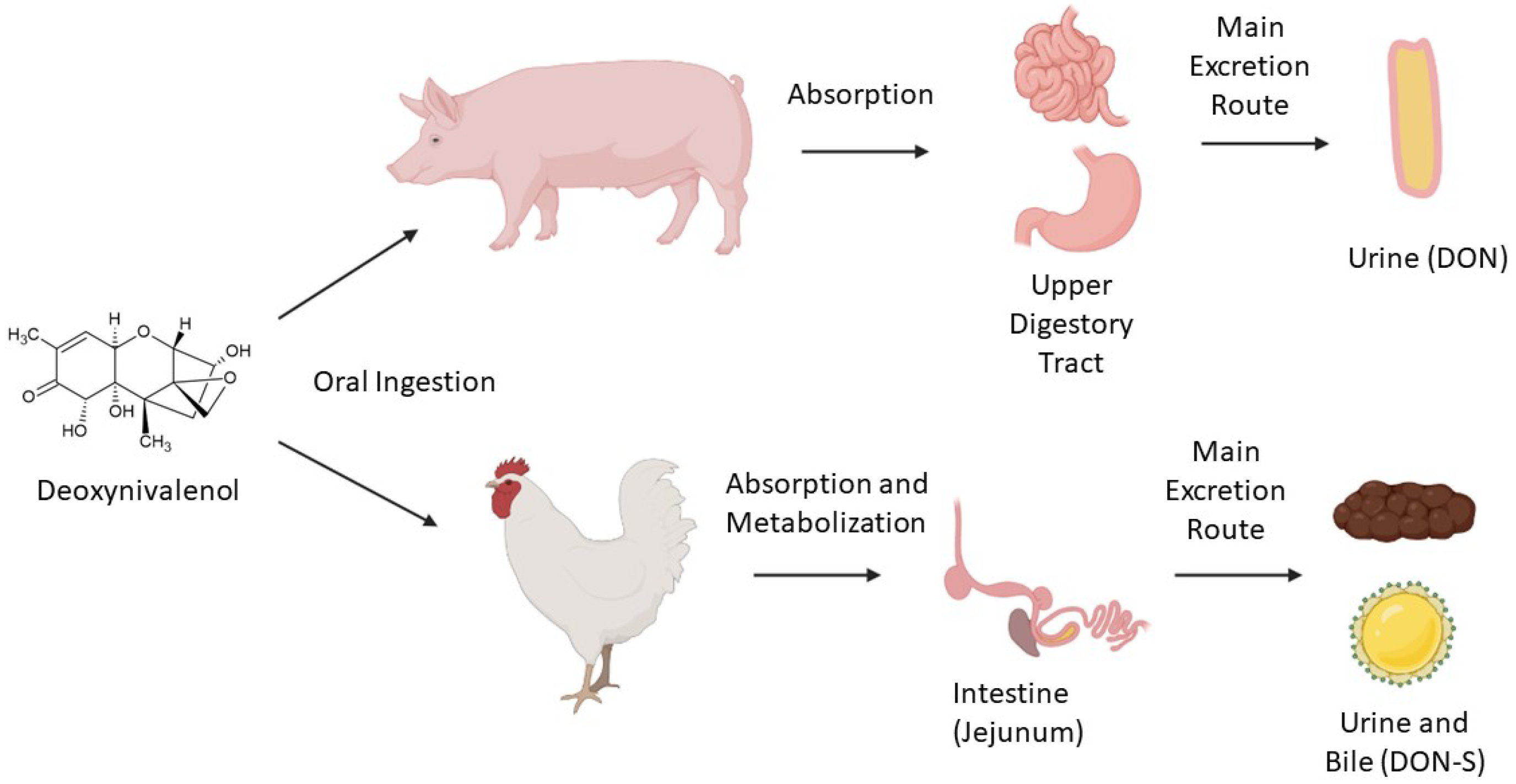
- Riahi, I.; Ramos, A.J.; Pérez-Vendrell, A.M.; Marquis, V. A toxicokinetic study reflecting the absorption, distribution, metabolism and excretion of deoxynivalenol in broiler chickens. J. Appl. Anim. Res. 2021, 49, 284–288.
- Paulick, M.; Winkler, J.; Kersten, S.; Schatzmayr, D.; Schwartz-Zimmermann, H.; Dänicke, S. Studies on the Bioavailability of Deoxynivalenol (DON) and DON Sulfonate (DONS) 1, 2, and 3 in Pigs Fed with Sodium Sulfite-Treated DON-Contaminated Maize. Toxins 2015, 7, 4622–4644.
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for Exposure as A Tool for Efficacy Testing of A Mycotoxin Detoxifier in Broiler Chickens and Pigs. Toxins 2019, 11, 187.
- Debevere, S.; Cools, A.; De Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity. Toxins 2020, 12, 101.
- Schelstraete, W.; Devreese, M.; Croubels, S. Comparative toxicokinetics of Fusarium mycotoxins in pigs and humans. Food Chem. Toxicol. 2020, 137, 111140.
- Guerre, P. Mycotoxin and Gut Microbiota Interactions. Toxins 2020, 12, 769.
- Korosteleva, S.N.; Smith, T.K.; Boermans, H.J. Effects of Feedborne Fusarium Mycotoxins on the Performance, Metabolism, and Immunity of Dairy Cows. J. Dairy Sci. 2007, 90, 3867–3873.
- van der Fels-Klerx, H.J.; Olesen, J.E.; Madsen, M.S.; Goedhart, P.W. Climate change increases deoxynivalenol contamination of wheat in north-western Europe. Food Addit. Contam. Part A 2012, 29, 1593–1604.
- Sundstøl Eriksen, G.; Pettersson, H.; Lundh, T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem. Toxicol. 2004, 42, 619–624.
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99.
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.-P.F.L.; Schatzmayr, G.; He, J.W.; Zhou, T.; Moll, W.-D.; et al. Microbial biotransformation of DON: Molecular basis for reduced toxicity. Sci. Rep. 2016, 6, 29105.
- Reddy, K.E.; Lee, W.; Jeong, J.Y.; Lee, Y.; Lee, H.-J.; Kim, M.S.; Kim, D.-W.; Yu, D.; Cho, A.; Oh, Y.K.; et al. Effects of deoxynivalenol- and zearalenone-contaminated feed on the gene expression profiles in the kidneys of piglets. Asian-Australas. J. Anim. Sci. 2018, 31, 138–148.
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877.
- Wang, X.; Fan, M.; Chu, X.; Zhang, Y.; Rahman, S.; Jiang, Y.; Chen, X.; Zhu, D.; Feng, S.; Li, Y.; et al. Deoxynivalenol induces toxicity and apoptosis in piglet hippocampal nerve cells via the MAPK signaling pathway. Toxicon 2018, 155, 1–8.
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786.
- Zhou, H.; Guog, T.; Dai, H.; Yu, Y.; Zhang, Y.; Ma, L. Deoxynivalenol: Toxicological profiles and perspective views for future research. World Mycotoxin J. 2020, 13, 179–188.
- Liu, M.; Zhang, L.; Chu, X.-H.; Ma, R.; Wang, Y.-W.; Liu, Q.; Zhang, N.-Y.; Karrow, N.A.; Sun, L.-H. Effects of deoxynivalenol on the porcine growth performance and intestinal microbiota and potential remediation by a modified HSCAS binder. Food Chem. Toxicol. 2020, 141, 111373.
- Wang, C.; Huang, L.; Wang, P.; Liu, Q.; Wang, J. The Effects of Deoxynivalenol on the Ultrastructure of the Sacculus Rotundus and Vermiform Appendix, as Well as the Intestinal Microbiota of Weaned Rabbits. Toxins 2020, 12, 569.
- Vatzia, E.; Pierron, A.; Hoog, A.M.; Saalmüller, A.; Mayer, E.; Gerner, W. Deoxynivalenol Has the Capacity to Increase Transcription Factor Expression and Cytokine Production in Porcine T Cells. Front. Immunol. 2020, 11, 2009.
- Cai, G.; Xia, S.; Zhong, F.; Liu, S.; Gu, J.; Yuan, Y.; Zhu, G.; Zou, H.; Liu, Z.; Bian, J. Zearalenone and deoxynivalenol reduced Th1-mediated cellular immune response after Listeria monocytogenes infection by inhibiting CD4+ T cell activation and differentiation. Environ. Pollut. 2021, 284, 117514.
- Kolesarova, A.; Medvedova, M.; Halenar, M.; Sirotkin, A.V.; Bulla, J. The influence of deoxynivalenol and zearalenone on steroid hormone production by porcine ovarian granulosa cells in vitro. J. Environ. Sci. Health Part B 2017, 52, 823–832.
- Gerez, J.R.; Desto, S.S.; Bracarense, A.P.F.R.L. Deoxynivalenol induces toxic effects in the ovaries of pigs: An ex vivo approach. Theriogenology 2017, 90, 94–100.
- Cao, Z.; Huang, W.; Sun, Y.; Li, Y. Deoxynivalenol induced spermatogenesis disorder by blood-testis barrier disruption associated with testosterone deficiency and inflammation in mice. Environ. Pollut. 2020, 264, 114748.
- Tassis, P.D.; Tsakmakidis, I.A.; Nagl, V.; Reisinger, N.; Tzika, E.; Gruber-Dorninger, C.; Michos, I.; Mittas, N.; Basioura, A.; Schatzmayr, D. Individual and Combined In Vitro Effects of Deoxynivalenol and Zearalenone on Boar Semen. Toxins 2020, 12, 495.
- Yang, J.; Wang, J.; Guo, W.; Ling, A.; Luo, A.; Liu, D.; Yang, X.; Zhao, Z. Toxic Effects and Possible Mechanisms of Deoxynivalenol Exposure on Sperm and Testicular Damage in BALB/c Mice. J. Agric. Food Chem. 2019, 67, 2289–2295.
- Wang, X.; Chen, X.; Cao, L.; Zhu, L.; Zhang, Y.; Chu, X.; Zhu, D.; Rahman, S.; Peng, C.; Feng, S.; et al. Mechanism of deoxynivalenol-induced neurotoxicity in weaned piglets is linked to lipid peroxidation, dampened neurotransmitter levels, and interference with calcium signaling. Ecotoxicol. Environ. Saf. 2020, 194, 110382.
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676.
- Bracarense, A.P.F.L.; Basso, K.M.; Da Silva, E.O.; Payros, D.; Oswald, I.P. Deoxynivalenol in the liver and lymphoid organs of rats: Effects of dose and duration on immunohistological changes. World Mycotoxin J. 2017, 10, 89–96.
- Ji, J.; Zhu, P.; Cui, F.; Pi, F.; Zhang, Y.; Sun, X. The disorder metabolic profiling in kidney and spleen of mice induced by mycotoxins deoxynivalenol through gas chromatography mass spectrometry. Chemosphere 2017, 180, 267–274.
- Huang, C.; Feng, L.; Liu, X.-A.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. The toxic effects and potential mechanisms of deoxynivalenol on the structural integrity of fish gill: Oxidative damage, apoptosis and tight junctions disruption. Toxicon 2020, 174, 32–42.
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A.; Bracarense, A.P.F.R.L. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon 2020, 185, 203–212.
- Wu, S.; Liu, Y.; Duan, Y.; Wang, F.; Guo, F.; Yan, F.; Yang, X.; Yang, X. Intestinal toxicity of deoxynivalenol is limited by supplementation with Lactobacillus plantarum JM113 and consequentially altered gut microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2018, 9, 74.
- Bracarense, A.P.F.L.; Pierron, A.; Pinton, P.; Gerez, J.R.; Schatzmayr, G.; Moll, W.-D.; Zhou, T.; Oswald, I.P. Reduced toxicity of 3-epi-deoxynivalenol and de-epoxy-deoxynivalenol through deoxynivalenol bacterial biotransformation: In vivo analysis in piglets. Food Chem. Toxicol. 2020, 140, 111241.
- Serviento, A.M.; Brossard, L.; Renaudeau, D. An acute challenge with a deoxynivalenol-contaminated diet has short- and long-term effects on performance and feeding behavior in finishing pigs. J. Anim. Sci. 2018, 96, 5209–5221.
- Rajput, S.A.; Shaukat, A.; Rajput, I.R.; Kamboh, A.A.; Iqbal, Z.; Saeed, M.; Akhtar, R.W.; Shah, S.A.H.; Raza, M.A.; El Askary, A.; et al. Ginsenoside Rb1 prevents deoxynivalenol-induced immune injury via alleviating oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112333.
- Wang, L.; Wang, Y.; Shao, H.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Luo, Y.; Zhang, D.; Chen, Z. In vivo toxicity assessment of deoxynivalenol-contaminated wheat after ozone degradation. Food Addit. Contam. Part A 2017, 34, 103–112.
- Yang, W.; Huang, L.; Wang, P.; Wu, Z.; Li, F.; Wang, C. The Effect of Low and High Dose Deoxynivalenol on Intestinal Morphology, Distribution, and Expression of Inflammatory Cytokines of Weaning Rabbits. Toxins 2019, 11, 473.
- Liu, D.; Wang, Q.; He, W.; Chen, X.; Wei, Z.; Huang, K. Two-way immune effects of deoxynivalenol in weaned piglets and porcine alveolar macrophages: Due mainly to its exposure dosage. Chemosphere 2020, 249, 126464.
- Alassane-Kpembi, I.; Canlet, C.; Tremblay-Franco, M.; Jourdan, F.; Chalzaviel, M.; Pinton, P.; Cossalter, A.M.; Achard, C.; Castex, M.; Combes, S.; et al. 1H-NMR metabolomics response to a realistic diet contamination with the mycotoxin deoxynivalenol: Effect of probiotics supplementation. Food Chem. Toxicol. 2020, 138, 111222.
- Guo, H.; Ji, J.; Wang, J.; Sun, X. Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 895–926.
- Wang, G.; Wang, Y.; Ji, F.; Xu, L.; Yu, M.; Shi, J.; Xu, J. Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 2019, 276, 436–442.
- He, J.W.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259.
- Vatzia, E.; Pierron, A.; Saalmüller, A.; Mayer, E.; Gerner, W. Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets. Toxins 2019, 11, 644.
- Mayer, E.; Novak, B.; Springler, A.; Schwartz-Zimmermann, H.E.; Nagl, V.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G. Effects of deoxynivalenol (DON) and its microbial biotransformation product deepoxy-deoxynivalenol (DOM-1) on a trout, pig, mouse, and human cell line. Mycotoxin Res. 2017, 33, 297–308.
