Today, more than ever, the search for non-trivial sources of biologically active substances is critical. Plants of the genus Rumex are noteworthy. Plants of this genus stand out for a number of advantages from the dominant plant core of meadow phytocenoses of the temperate climatic zone: a short growing season, an intensive increase in biomass, and undemanding growth conditions. In addition, this plant genus is known as a super-producer of secondary phenolic compounds.
- sorrel
- dock
- ethnobotany
- medicinal plants
- edible plants
- chemotaxonomy
- phylogeny
- secondary metabolites
- superfood
- raw material
1. Introduction
2. Variation in the Content of Some Groups of Phenolic Compounds
| Species | TPC 1, mg GAE g–1 |
TFC, mg RE g–1 |
THA, mg CAE g–1 |
TCC, mg CE g–1 |
PAs, mg CyE g–1 |
TTC, mg GAE g–1 |
|---|---|---|---|---|---|---|
| R. acetosa | 23 ± 2 | 18 ± 1 | 12.7 ± 0.6 | 0.90 ± 0.05 | 0.24 ± 0.02 | 0.46 ± 0.05 |
| R. acetosella | 117 ± 7 | 106 ± 4 | 18 ± 1 | 1.3 ± 0.1 | 2.2 ± 0.2 | 11 ± 1 |
| R. confertus | 76 ± 7 | 38± 2 | 4.8 ± 0.3 | 5.0 ± 0.3 | 4.0 ± 0.3 | 6.4 ± 0.3 |
| R. crispus | 131 ± 10 | 92± 5 | 8.9 ± 0.6 | 5.2 ± 0.3 | 6.4 ± 0.3 | 14 ± 1 |
| R. maritimus | 111 ± 6 | 120 ± 9 | 5.8 ± 0.6 | 4.8 ± 0.3 | 5.0 ± 0.4 | 7.1 ± 0.6 |
| R. obtusifolius | 129 ± 9 | 92 ± 4 | 1.9 ± 0.1 | 6.0 ± 0.4 | 7.2 ± 0.5 | 17 ± 1 |
| R. sanguineus | 126 ± 5 | 99 ± 6 | 1.9 ± 0.1 | 10.9 ± 0.6 | 6.6 ± 0.4 | 12.9 ± 0.7 |
A high content of flavonoids was characteristic of the leaves of R. maritimus and R. acetosella (Table 1). The total flavonoids content in the leaves of R. crispus, R. obtusifolius, and R. sanguineus varied from 92 to 98 mg g−1. A notably lower content of flavonoids was found in the leaves of R. confertus (about 38 mg g−1) and R. acetosa (about 18 mg g−1).
The hydroxycinnamic acids’ accumulation in the leaves of the studied species showed a somewhat different tendency (Table 1). The highest content was found in the leaves of R. acetosella (about 18 mg g−1). However, as opposed to the TPC and TFC values, the leaves of R. acetosa were characterized by a high total content of hydroxycinnamic acids as well (up to 13 mg g−1). Whereas the leaves of R. obtusifolius and R. sanguineus did not show THA values higher than 2 mg g−1 (Table 1).
The highest total catechins content was found in the leaves of R. sanguineus—about 11 mg g−1 (Table 1). The TCC values of R. obtusifolius, R. confertus, R. crispus, and R. maritimus leaves were almost twice as low (from 4.8 to 6 mg g−1). The R. acetosa and R. acetosella leaves demonstrated the lowest catechin content (from 0.9 to 1.3 mg g−1).
The leaves of R. sanguineus, R. obtusifolius, and R. crispus were shown to have a high amount of proanthocyanids (from 6.4 to 7.2 mg g−1). The lowest PA content was found in the leaves of R. acetosa (0.24 mgg−1) (Table 1). The leaves of R. acetosa were also characterized by a very low content of tannins (less than 0.5 mg g−1), while the highest level of TTC was found in the leaves of R. obtusifolius (about 17 mg g−1) (Table 1).
Thus, various species of Rumex were associated with their own maxima of individual phenolic group levels: R. maritimus—flavonoids, R. acetosella—hydroxycinnamic acids, R. sanguineus—catechins, R. sanguineus, R. obtusifolius, R. crispus—proanthocyanidins, R. obtusifolius —tannins. The leaves of R. acetosa were characterized by the lowest contents of all analyzed phenolic compounds, except for the THA level (Table 1).
3. Variation in the Content of Individual Phenolic Compounds
Despite the low value of TPC, R. acetosa demonstrated a remarkable diversity of phenolic compounds, especially phenolic acids (Table 2; Appendix A, Figure A1a). The leaves of R. acetosa contained protocatechuic acid, sinapic acid, caftaric acid, chlorogenic acid, p-coumaric acid, ellagic acid, and other hydroxybenzoic acid derivatives. Among them, sinapic acid was the most present (about 5 mgg−1). Moreover, multiple types of flavonoids, such as derivatives of quercetin (rutin, isoquercitrin, and so son) and luteolin (cynaroside), were found in the leaves.
| Compounds (Retention Time, Min) |
Content of Individual Phenolic Compounds, mg g–1 | ||||||
|---|---|---|---|---|---|---|---|
| R. acetosa | R. acetosella | R. confertus | R. crispus | R. maritimus | R. obtusifolius | R. sanguineus | |
| Flavonoids | |||||||
| Catechin (9.7) | – | – | – | 1.08 ± 0.07 | 0.17 ± 0.01 | 1.32 ± 0.07 | 12.0 ± 0.8 |
| Quercetin 3-O-rutinoside (rutin) (19.3) | 3.4 ± 0.2 | – | 4.3 ± 0.2 | 10.2 ± 0.7 | 9.4 ± 0.6 | 19.0 ± 1.1 | 8.6 ± 0.5 |
| Quercetin 3-β-D-glucoside (isoquercitrin) (19.9) | 0.56 ± 0.03 | – | 20.2 ± 1.3 | 31.9 ± 1.8 | 22.6 ± 1.5 | 54.8 ± 3.5 | 49.5 ± 0.3 |
| Quercetin derivative (16.3)1 | – | – | – | – | 39.6 ± 2.9 | – | – |
| Quercetin derivative (16.9) | – | – | – | – | 27.1 ± 1.5 | – | – |
| Quercetin derivative (18.3) | 2.4 ± 0.2 | – | 0.94 ± 0.06 | – | – | – | – |
| Quercetin derivative (22.73) | 1.31 ± 0.07 | – | |||||
| – | |||||||
| 2.9 ± 0.2 | |||||||
| – | |||||||
| – | |||||||
| Hydroxybenzoic acid | |||||||
| derivative (12.5) | |||||||
| 0.54 ± 0.05 | |||||||
| 3.0 ± 0.2 | |||||||
| – | |||||||
| – | – | – | – | ||||
A characteristic feature of R. acetosella was the presence of mostly flavones (derivatives of luteolin and apigenin) in the leaves, in contrast to other species, where flavonols (derivatives of quercetin and kaempferol) prevailed (Table 2; Appendix A, Figure A1b). Moreover, R. acetosella was characterized by a diverse composition and a high content of phenolic acids. The leaves are shown to contain protocatechuic acid, sinapic acid, chlorogenic acid, caffeic acid, and other derivatives of hydroxybenzoic acids.
The leaves of R. confertus, R. crispus, R. maritimus, R. obtusifolius, and R. sanguineus showed the presence of rutin and isoquercitrin, the content ratio of which varied in these species from 1:2.5 to 1:5.8, as well as the presence of astragaline and another kaempferol derivative (Table 2; Appendix A, Figure A1c–g). In fact, R. confertus, R. crispus, R. obtusifolius, and R. sanguineus demonstrated a higher level of isoquercitrin compared with other phenolic compounds present in the leaves.
The leaves of R. crispus were characterized by a high content of kaempferol derivatives (about 37 mg g−1 in total) and gallic acid (about 5 mg g−1) compared with other studied species (Table 2; Appendix A, Figure A1d).
The R. maritimus sample showed the highest concentration of quercetin derivatives (Table 2). Moreover, this species was characterized by a rich qualitative composition of phenolic acids. It includes protocatechuic acid, sinapic acid, chlorogenic acid, caffeic acid, and other derivatives of hydroxybenzoic acids.
The leaves of R. obtusifolius and R. sanguineus had a similar metabolic profile with high levels of flavonoids (quercetin derivatives) and very low levels of phenolic acids (Table 2; Appendix A, Figure A1f,g). However, it should be noted that the leaves of R. sanguineus were high in catechin (up to 12 mg g−1), in contrast to R. obtusifolius and other analyzed species.
4. Antioxidant Activity of the Rumex Extracts
Extracts from R. crispus demonstrated high antioxidant activity based on all three methods (Table 3). A high level of antioxidant activity was also found in R. maritimus extracts (according to the ABTS and FRAP methods). The lowest antioxidant activity was shown by the extracts of R. acetosa (Table 3).
| Species | AOA (DPPH) 1, mg TE g–1 |
AOA (ABTS), mg TE g–1 |
AOA (FRAP), mg TE g–1 |
|---|---|---|---|
| R. acetosa |
Antioxidant activity is caused by the presence of certain components in plant samples, usually compounds of phenolic nature. Correlation analysis carried out during this research proved a positive relationship between antioxidant activity and the total content of phenolic compounds (r = 0.785–0.921, p ≤ 0.05), flavonoids (r = 0.602–0.918, p ≤ 0.05), proanthocyanidins (r = 0.721–0.842, p ≤ 0.05), and tannins (r = 0.591–0.776, p ≤ 0.05) (Table 4). However, the results related to the content of hydroxycinnamic acids were unexpected. Either there was no significant correlation between the antioxidant activity level (according to the DPPH and ABTS methods) and THA, or there was an inverse correlation of moderate strength (when based on the FRAP method).
| Variables | TPC 1 | TFC | THA | TCC | PAs | TTC | DPPH | ABTS | FRAP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.1 ± 0.3 | 5.1 ± 0.5 | 3.9 ± 0.3 | |||||||||||||||
| TPC | 1 | 0.881 ** | –0.317 ns | 0.567 * | 0.822 ** | 0.915 ** | 0.806 ** | 0.921 ** | 0.785 ** | ||||||||
| R. acetosella | TFC31 ± 3 | 48 ± 3 | 27 ± 3 | ||||||||||||||
| – | |||||||||||||||||
| 1 | –0.114 | ns | 0.368 | ns | 0.586 * | 0.664 * | 0.602 * | 0.918 ** | 0.714 ** | R. confertus | 22 ± 1.3 | ||||||
| THA | 37 ± 3 | 39 ± 4 | |||||||||||||||
| 1 | –0.820 ** | –0.768 ** | –0.354 | ns | –0.174 | ns | –0.322 | ns | –0.563 * | R. crispus | 69 ± 4 | 56 ± 4 | 57 ± 3 | ||||
| TCC | 1 | 0.809 ** | 0.537 * | 0.389 ns | 0.513 * | 0.614 * | R. maritimus | 31 ± 2 | 63 ± 4 | 61 ± 4 | |||||||
| PAs | 1 | 0.826 ** | 0.721 ** | 0.751 ** | 0.842 ** | R. obtusifolius | |||||||||||
| TTC | 37 ± 2 | 48 ± 4 | 43 ± 2 | ||||||||||||||
| R. sanguineus | 35 ± 3 | 52 ± 4 | 47 ± 4 | – | – | – | – | ||||||||||
| Quercetin derivative (23.1) | 2.0 ± 0.1 | – | – | – | – | – | – | ||||||||||
| 1 | 0.776 ** | 0.701 ** | 0.591 * | ||||||||||||||
| DPPH | 1 | 0.728 ** | 0.742 ** | ||||||||||||||
| ABTS | Quercetin derivative (24.1) | 3.4 ± 0.2 | – | – | – | – | – | – | |||||||||
| Kaempferol 3-O-glucoside (astragalin) (24.7) | – | – | 1.82 ± 0.09 | 24.4 ± 1.6 | 4.4 ± 0.3 | 5.1 ± 0.3 | 8.6 ± 0.6 | ||||||||||
| Kaempferolderivative (22.8) | – | – | 0.75 ± 0.04 | 12.9 ± 1.0 | 1.4 ± 0.1 | 2.6 ± 0.2 | 3.2 ± 0.2 | ||||||||||
| 1 | 0.909 ** | ||||||||||||||||
| FRAP | 1 | Kaempferolderivative (20.9) | – | – | – | – | 4.9 ± 0.3 | – | – | ||||||||
| Luteolin 7-O-glucoside (cynaroside) (20.7) | 0.51 ± 0.03 | 4.3 ± 0.3 | – | – | – | – | – | ||||||||||
| Luteolinderivative (15.5) | – | 89.5 ± 4.7 | – | – | – | – | – | ||||||||||
| Apigeninderivative (19.4) | – | 5.1 ± 0.3 | |||||||||||||||
| Phenolic acids | |||||||||||||||||
| Gallic acid (3.8) | – | – | – | 5.3 ± 0.3 | – | 0.34 ± 0.02 | 0.33 ± 0.02 | ||||||||||
| 3,4-Dihydroxybenzoic acid (protocatechuic acid) (5.8) | 0.12 ± 0.01 | 0.58 ± 0.03 | – | 0.56 ± 0.03 | 0.21 ± 0.01 | – | 0.21 ± 0.01 | ||||||||||
| Sinapic acid (8.2) | 4.9 ± 0.4 | 1.22 ± 0.08 | 1.5 ±0.1 | – | 1.8 ± 0.1 | – | – | ||||||||||
| Caftaric acid (9.2) | 1.7 ± 0.1 | – | – | – | – | – | – | ||||||||||
| Chlorogenic acid (10.2) | 1.21 ± 0.09 | 3.04 ± 0.17 | 1.8 ± 0.1 | – | 0.19 ± 0.01 | – | – | ||||||||||
| Caffeic acid (10.5) | – | 0.93 ± 0.05 | – | 0.10 ± 0.01 | 0.29 ± 0.03 | – | – | ||||||||||
| p-Coumaric acid (14.2) | 0.15 ± 0.02 | – | – | – | – | – | – | ||||||||||
| Ellagic acid (17.9) | 0.28 ± 0.02 | – | – | 0.83 ± 0.05 | – | – | – | ||||||||||
| Hydroxybenzoic acid derivative (11.2) | 0.97 ± 0.05 | 4.0 ± 0.2 | – | ||||||||||||||
5. Correlation between Phenolic Compounds Content and Antioxidant Activity
1 TPC, total phenolics content; TFC, total flavonoids content; THA, total hydroxycinnamic acids; TCC, total catechins content; PAs, total proanthocyanidins content; TTC, total tannins content; DPPH, antioxidant activity determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay; ABTS, antioxidant activity determined by the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay; FRAP, ferric reducing antioxidant power. ** Correlation is significant at p ≤ 0.01; * correlation is significant at p ≤ 0.05; ns, correlation is not significant (p > 0.05).
6. Heat Map and Cluster Analysis of Studied Rumex Species Based on the Content of Phenolic Compounds and Antioxidant Activity of Their Extracts
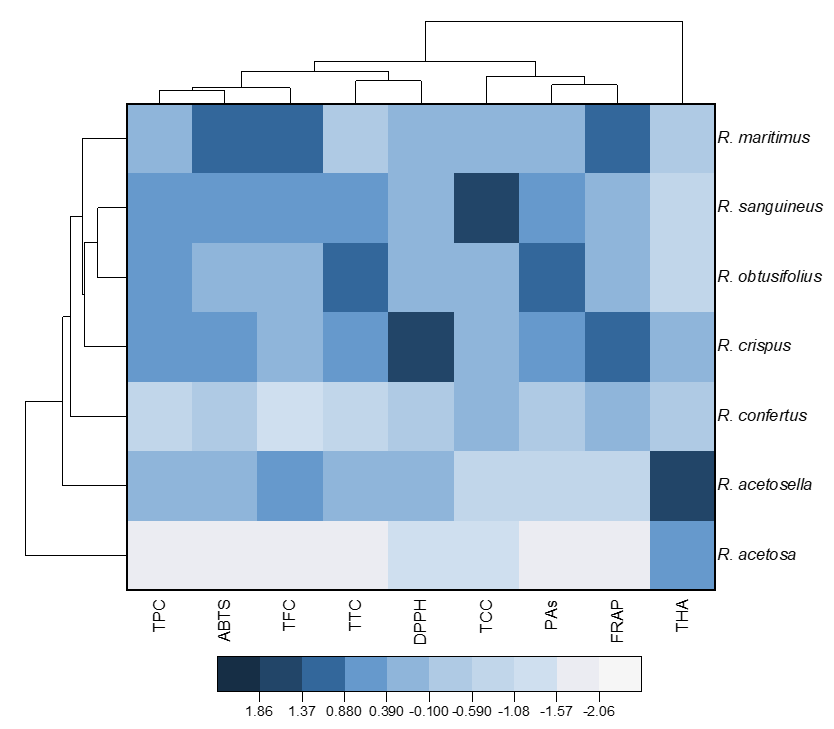
Figure 1. Heat map with clusters for studied variables (at the top) and Rumex species (at the left). TPC, total phenolics content; TFC, total flavonoids content; THA, total hydroxycinnamic acids; TCC, total catechins content; PAs, total proanthocyanidins content; TTC, total tannins content; DPPH, antioxidant activity determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay; ABTS, antioxidant activity determined by the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay; FRAP, ferric reducing antioxidant power.
The dendrogram on the left shows that the analyzed Rumex species can be divided into two large clusters (Figure 1). The first of them consists of only R. acetosa, and the second of all the other studied species. The second cluster includes multiple groups. One of them has only R. acetosella, whereas the other group includes R. sanguineus, R. obtusifolius, R. crispus, and R. confertus. The dendrogram shows that, in the latter group, R. sanguineus and R. obtusifolius in turn form a micro-group characterized by very similar composition.
Appendix A
References
- Carvalho, A.M.; Barata, A.M. The consumption of wild edible plants. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; Ferreira, I.C.F.R., Morales, P., Barros, L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 159–198.
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New Functional Crops”? Molecules 2018, 23, 2299.
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium Biofortification of Three Wild Species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., Grown as Microgreens. Agronomy 2021, 11, 1155.
- Faehnrich, B.; Franz, C.; Nemaz, P.; Kaul, H.-P. Medicinal plants and their secondary metabolites—State of the art and trends in breeding, analytics and use in feed supplementation—with special focus on German chamomile. J. Appl. Bot. Food Qual. 2021, 94, 61–74.
- Migicovsky, Z.; Myles, S. Exploiting Wild Relatives for Genomics-assisted Breeding of Perennial Crops. Front. Plant Sci. 2017, 8, 460.
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild vegetable Rumex acetosa Linn.: Its ethnobotany, pharmacology and phytochemistry—A review. S. Afr. J. Bot. 2019, 125, 149–160.
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228.
- Podgurskaya, V.V.; Luksha, E.A.; Gushchina, E.S.; Savchenko, I.A.; Korneeva, I.N.; Kalinkina, G.I. Biological activity of the genus Rumex (Polygonaceae) plants. Chem. Plant Raw Mater. 2021, 2, 59–78.
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C.F.R. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188.
- Wegiera, M.; Smolarz, H.D.; Wianowska, D.; Dawidowicz, A.L. Anthracene derivatives in some species of Rumex L. genus. Acta Soc. Bot. Pol. 2007, 76, 103–108.
- Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. American Herbal Pharmacopoeia: Botanical Pharmacognosy-Microscopic Characterization of Botanical Medicines; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1420073281.
- Gosudarstvennaya Farmakopeya Rossiyskoy Federatsii XIV izdaniye. In ; Emshanova, S.V.; Potanina, O.G.; Budanova, E.V.; Chistyakov, V.V. (Eds.) Ministry of Health of Russian Federation: Moscow, Russia, 2018; Volume 4, p. 1844. (In Russian)
- Shaikh, S.; Shriram, V.; Srivastav, A.; Barve, P.; Kumar, V. A critical review on Nepal Dock (Rumex nepalensis): A tropical herb with immense medicinal importance. Asian Pac. J. Trop. Med. 2018, 11, 405–414.
- Prakash Mishra, A.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34.
- Li, Y.; Jiang, J.-G. Health functions and structure-activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6063–6080.
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Diaz, M.A.N. Chapter 11—Anthraquinones: An overview. In Studies in Natural Products Chemistry; Atta-ur-Rahman, B.T.-S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 313–338. ISBN 1572-5995.
- Balog, K.; Svirčev, E.; Lesjak, M.; Orcic, D.; Beara, I.; Francišković, M.; Simin, N. Phenolic profiling of Rumex L. species by means of the LC-MS/MS. Planta Med. 2012, 78, PL12.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93.
