Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 3 by Nora Tang.
To take advantage of ROS-specific cleavage, different technological uses of Thioketal (TK) functional group have been proposed in the design of selective and improved “smart” nanosystems, including as linker to connect a drug on the surface of nanoparticles, form prodrugs, as a core component of the Drug Delivery System (DDS) to directly control its structure, to control the opening of drug-releasing gates or to change the conformation of the nano-systems. This allows to overcome the major limitations of conventional DDS counterparts, including uncontrolled drug release and off-target effects, by controlling the degradation kinetics, drug release kinetics, or even increasing potential administration routes.
- thioketal
- smart drug delivery systems
- ROS-responsive biomaterials
- nanomedicine
- nanoparticles
1. TK Used as a Linker in DDS Conjugation for On-Demand Drug Release
One possible use of thioketal (TK) in designing ROS-specific DDS consists of covalently attaching it to drug, dye, or biologically active molecules to obtain a linker that causes its release upon ROS-specific cleavage. In this way, therapeutic efficacy can be improved with minimized off-target effects by releasing the conjugated drug in a controlled manner [1].
1.1. TK as Linker to Attach Drugs to the Surface of Nanoparticles
As a first approach, TK linkers have been exploited for the covalent attachment of drugs to the surface of inorganic NPs (Figure 1a). For instance, Shi et al. developed a novel surface-functionalized fullerene (C60)-based DDS targeted to tumors with the Asn-Gly-Arg peptide designed to with an “off-on” state to maximize the antitumor efficacy of the nanosystem. In this work, doxorubicin was attached to C60 via a ROS-sensitive thioketal linker. When in its “off”-state the doxorubicin remained firmly bound to the surface while light activation (the “on”-state) led to ROS production, the breakdown of the TK linker and subsequent drug release [2].
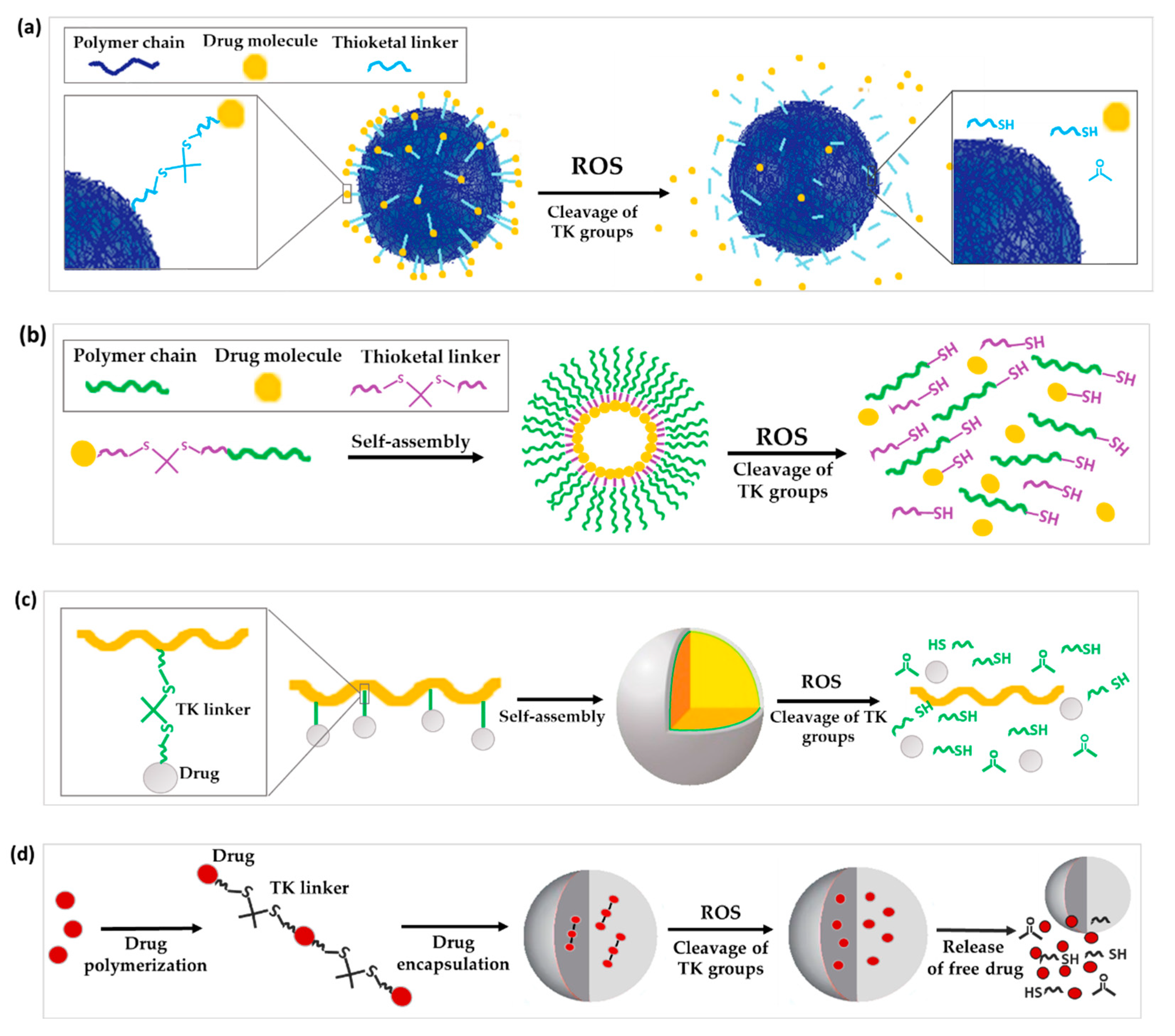
Figure 1. Schematic illustration of the chemical structure and corresponding release mechanisms of TK-based ROS-responsive nanosystems, where TK is used as (a) linker to attach drugs to the surface of nanoparticles, (b) linkage to form prodrug-based NPs, (c) linker to form polyprodrug-based NPs with drug molecules attached to the same polymer unit by TK linkages or (d) linker to form encapsulated polyprodrugs based on multiple drug molecules attached to each other via TK bridges.
1.2. TK as a Linker for Polymer-Dye Conjugation
Alternatively, TK has been used as a linker to form polymer-dye conjugates. Recently, the synthesis of a fluorescent conjugate between Cy5, a near-infrared fluorescence-emitting dye, the TK moiety, and polyethylene glycol monomethyl ether (mPEG) (mPEG-TK-Cy5) was reported. The aim was to perform proof-of-concept studies that confirm the selective release of this dye in cancer cells respective of normal cells. In ROS solutions it was observed that the presence of TK blocked Cy5 quenching. Further in vitro studies using rat glioblastoma cells (C6) with high intrinsic levels of ROS demonstrated the release of Cy5, while no release was achieved in normal astrocyte cells with physiological levels of ROS (DI TNC1) [3].
1.3. TK as Linkage to Form Prodrug or Polyprodrugs
Prodrugs are inactive precursors of a drug designed to increase the amount of drug converted to its active form by a certain pathological or biological stimulus [4][5][6]. With the prodrug idea in mind, ROS-sensitive TK linkers have been exploited to conjugate a drug to another drug or polymer that can then self-assemble into NPs [7]. Once the prodrug NPs reach the diseased tissues, the high concentration of ROS cleaves the TK linker to release and activate the drug (Figure 1b).
In order to treat inflammatory bowel disease, Li et al. used an aromatized TK to conjugate budesonide (an anti-inflammatory drug [8]) with the antioxidant Tempol to improve colon delivery. Interestingly, π-π stacking interactions of the TK linker, as well as hydrophobic interactions between budesonide moieties promoted self-assembly of the prodrug into NPs. In fact, authors were able to calculate the critical aggregation concentration of the prodrug as 16 µg/mL, which is low thanks to the strong non-covalent hydrophobic interactions. Ultimately, hydroxide-triggered cleavage of TK activated the drug and achieving anti-inflammatory, antioxidant, and anti-apoptotic effects in an inflammatory bowel disease mouse model [9]. Other examples regarding TK-containing prodrugs included improving the solubility and pharmacokinetic half-life of chemotherapeutic drugs by conjugating them to polyethylene glycol (PEG) [10]. For instance, melphalan (MPH) was conjugated to PEG via TK to form a prodrug that self-assembled into micelles for treating glioblastoma multiforme. The incorporation of the TK linker enabled selective ROS-triggered release of MPH in cancer cells increasing the anticancer activity and cytotoxicity in rat C6 and human U251 MG glioblastoma cells compared to the non-ROS sensitive prodrug mPEG-MPH. On the other hand, TK was not cleaved and did not induce cytotoxicity in healthy DI TNC1 cells [7]. In another study, Pan et al. synthesized a PEG-doxorubicin (DOX) conjugate via a TK moiety that self-assembled into prodrug NPs that showed negligible release of DOX in HepG2 cells but significantly enhanced drug release (45.5% in 24 h) in the presence of 100 μM H2O2, indicating the tumor-ROS-responsiveness of NPs selectively in ROS-rich environment. Furthermore, these nanosystems showed higher antitumor activity than free DOX in liver cancer (HepG2)-bearing nude mice [11]. In addition, Lin et al. synthesized a PEG-TK-mitoxantrone prodrug that co-assembled with lipid-PEG to form NPs embedding a cisplatin prodrug in the core for combined cancer therapy. Release studies showed that about 80% of cisplatin prodrug and 40% of the loaded mitoxantrone (MTO) were rapidly released from NPs within 24 h when incubated in PBS solution containing 100 μM KO2, which is a biologically relevant intracellular ROS concentration. Moreover, when the TK group was replaced with a non-responsive carbon–carbon bond no drug release was observed from the NPs at 100 μM KO2, and no difference was observed in their release profile compared to NPs incubated without ROS. In vivo, the environmental responsive cleavage of the TK led to localized release of both the MTO and cisplatin creating a synergistic tumor growth inhibition in a prostate cancer mice model [12].
TK linkers have also been applied for the development of polyprodrugs where: (a) drug molecules are attached to the same polymer unit by TK linkages or (b) drug molecules are attached to each other by means of TK bridges (Figure 1c,d) [13][14]. Regarding the first type of polyprodrug, Yin and co-workers conjugated camptothecin (CPT) molecules to co-block polymers from PEG and polymerized methacrylate monomers by linking the drug to the side chains of methacrylate units through a TK linker. These co-block polymers self-assembled into micelles encapsulating β-lapachone (Lapa), named Lapa@NPs, for cancer application [15]. Regarding the second type of polyprodrug, Xu and co-workers polymerized multiple molecules of MTO by TK linkers. This polyprodrug self-assembled into NPs which were further surface decorated with the RGD (arginine-glycine-aspartic acid) peptide to impart tumor-targeting ability. These NP- prodrugs showed high and stable drug loading. The polymerization of MTO prevented the escape of drug molecules from NPs, and the absence of ROS led to negligible drug release in vitro. In contrast, 25% and 40% of loaded MTO was released from NPs when incubated in PBS buffer containing 50 μM and 100 μM of KO2 solution for 48 h, respectively. TK bonds in the structure of the polyprodrug ensured a ROS-dependent chain-breakage and the consequent release of active MTO in cancer cells [16].
2. TK Incorporated into the Polymer Structure
A major goal of nanomedicine is to develop stable nanocarriers that protect a drug during its passage through the bloodstream and release the payload in the diseased site via controlled degradation of the DDS. Although such a design concept is well known, most of the conventional nanocarriers do not adequately fulfill these requirements, resulting in premature and/or off-target drug release [17]. TK’s specificity for being degraded by ROS, and the higher ROS concentrations in several diseased states, makes it a prime candidate. In this regard, the incorporation of a ROS-sensitive TK moiety into the polymer backbone of the nanosystem has emerged as a promising strategy to control degradation and release kinetics, and to release the payload specifically in the diseased area. In the reported studies, controlled degradation and drug release normally occur by ROS-triggered cleavage of the TK chemical bonds leading to polymer backbone decomposition.
To fulfill these objectives, some works reported the incorporation of TK linkages into homopolymers. For instance, Kim et al. synthesized poly(1,4-phenyleneacetone dimethylene thioketal) polymer (PPADT). PPADT was used to prepare polymeric NPs loaded with Nile red as a model drug. The molecular weight of PPADT dramatically decreased to 28.8% over 24 h after incubation in a 10 mM KO2 solution. This is compared to 92% of the polymer remaining intact over 96 h in the absence of the TK linker. While the drug released from the NPs was negligible in the absence of ROS after 60 h, it reached 73% in presence of TK and 10 mM ROS. Furthermore, no significant change in the hydrodynamic diameter of the NPs was observed during exposure to ROS. This suggests that the degradation release of the drug was not caused by the destruction of the core, but rather by holes within the matrix caused by the destruction of the polymer backbone bonds (Figure 2a) [18]. Similar results were observed in two other studies by Wang et al. and Wilson and co-workers. PPADT was exploited to develop NPs loaded with stromal cell-derived factor 1α (SDF-1α), a chemokine that plays a key role in wound treatment, or a small oligonucleotide (siRNA) that inhibits the expression of the proinflammatory cytokine tumor necrosis factor-α (TNFα) [19][20]. All three of these drug-loaded NPs displayed significantly higher therapeutic effectiveness in vivo compared to nanoparticles without TK.
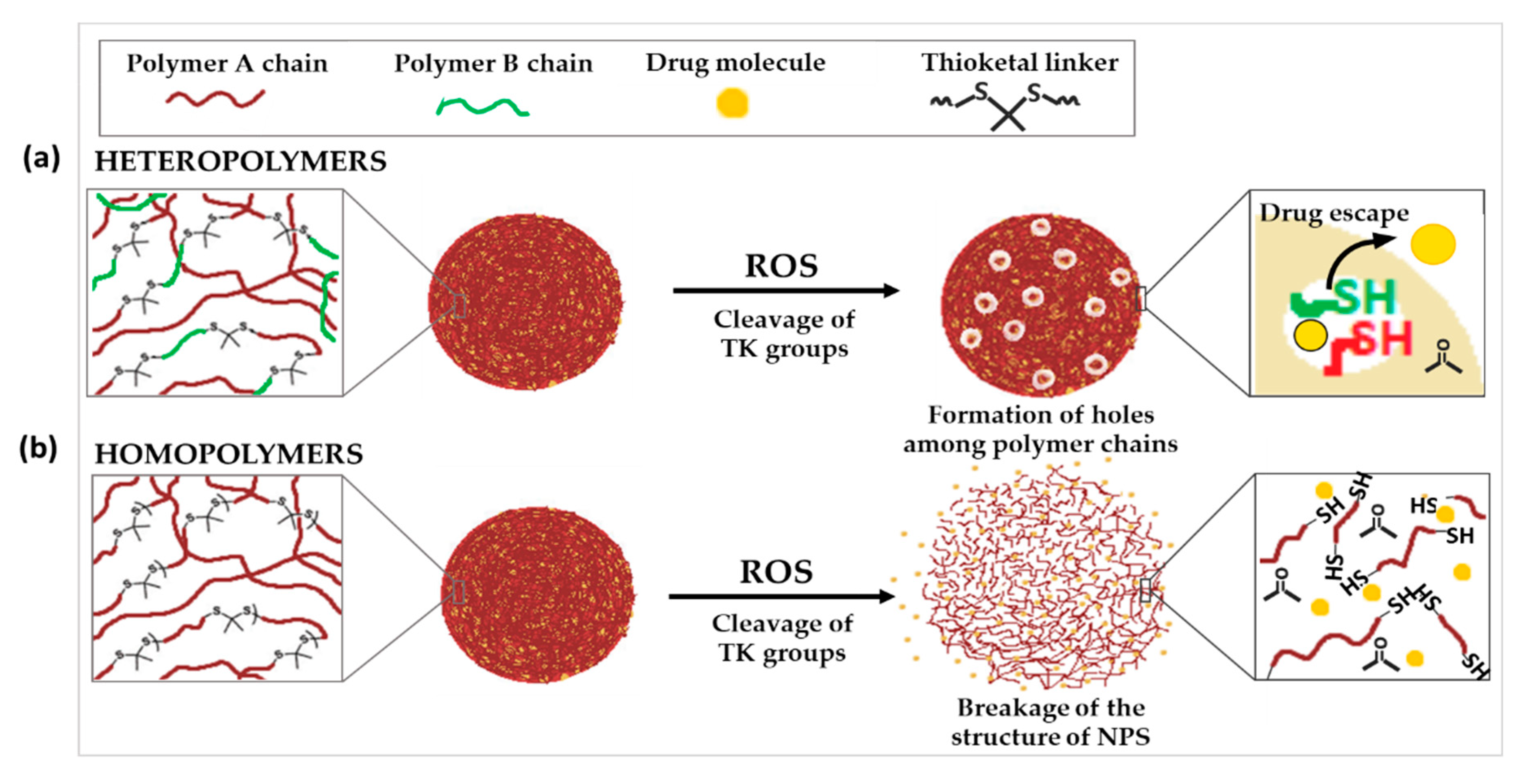
Figure 2. Schematic illustration of the chemical structure and corresponding release mechanisms of TK-based ROS-responsive nanosystems, where TK is incorporated into (a) heteropolymers, two polymers with different physico-chemical characteristics or (b) homopolymers.
As an alternative to the incorporation of TK into homopolymers, it has also been used to link together two polymers with different physico-chemical characteristics. In contrary to the previous cases, where the ROS-triggered cleavage of TK groups did not cause a significant change in the size of NPs, other examples demonstrated huge increase in nanoparticle diameter after exposure to ROS. This could suggest that the cleavage of TK resulted in the complete breakdown of the NP structure (Figure 3b).
Two examples can be found in the studies carried out by Li et al. In the first study, they conjugated poly (lactic-co-glycolic acid) (PLGA) with methoxyl-polyethylene glycol (MeO-PEG) by TK linker to form a diblock co-polymer that self-assembled into NPs loaded with DOX. In the second study, MeO-PEG was replaced by PEG and DOX was co-loaded into NPs with α-tocopheryl succinate, which stimulates ROS production into cells. In both cases, the 6 h and 48 h cumulative release of DOX from NPs was significantly higher when NPs were incubated with 50 μM ROS, which increased significantly to more than 80% after 48 h when incubated in 100 μM. This increase in DOX release rate was attributed to the cleavage of TK linker resulting in the breakdown of the NPs increasing their size and releasing DOX. In contrast, no morphological changes were detected in the absence of ROS or in control drug-loaded NPs without the TK linker. Moreover, co-loading alpha-tocopheryl succinate with DOX led to enhanced antitumor efficiency, mainly due to the higher intracellular ROS levels in cancer cells induced by alpha-tocopherol which promoted the cleavage of TK [21][22]. In another work, Sun et al. used a TK linker with a π-conjugated structure between methoxy polyethylene glycol (PEG) and poly(ε-caprolactone) (PCL) chains. mPEG-TK-PCL self-assembled into micelles which were loaded with doxorubicin. π–π stacking and hydrophobic interactions between TK moieties and DOX enhanced the drug loading content of micelles (12.8%) with respect to mPEG-PCL micelles without TK linker (8.6%). While the size of DOX-loaded micelles remained unchanged in PBS, it increased to more than 1000 nm when the micelles were put in ROS-rich microenvironment for 48 h. This increase in size could be attributed to the ROS-responsive degradation of the polymer leading to breakdown and swelling of the core structure with subsequent release of DOX. This selective ROS-responsive drug release helped lower in vivo toxicity towards normal cells and remarkably enhanced antitumor efficacy of DOX-loaded micelles [23].
3. Other Technological Uses of TK in DDS Structure
Along with releasing a conjugated pharmaco or destabilizing the DDS structure to control release, other interesting technological uses of TK have emerged. Among them, TK-based biomaterials have been used to control the opening of drug-release gates, or to change the surface characteristics or the conformation of NPs upon ROS exposure.
3.1. TK-Based ROS-Responsive Gates to Prevent Premature Drug Release
Stimuli-responsive gatekeepers are structures able to keep the pores of NPs closed under physiological conditions and open them on-demand after the application of a particular stimulus, in this case the upregulated ROS levels in the pathological site [24][25]. The use of ROS-responsive gates based on TK has been proposed to prevent the premature release of drugs from NPs (Figure 3a).
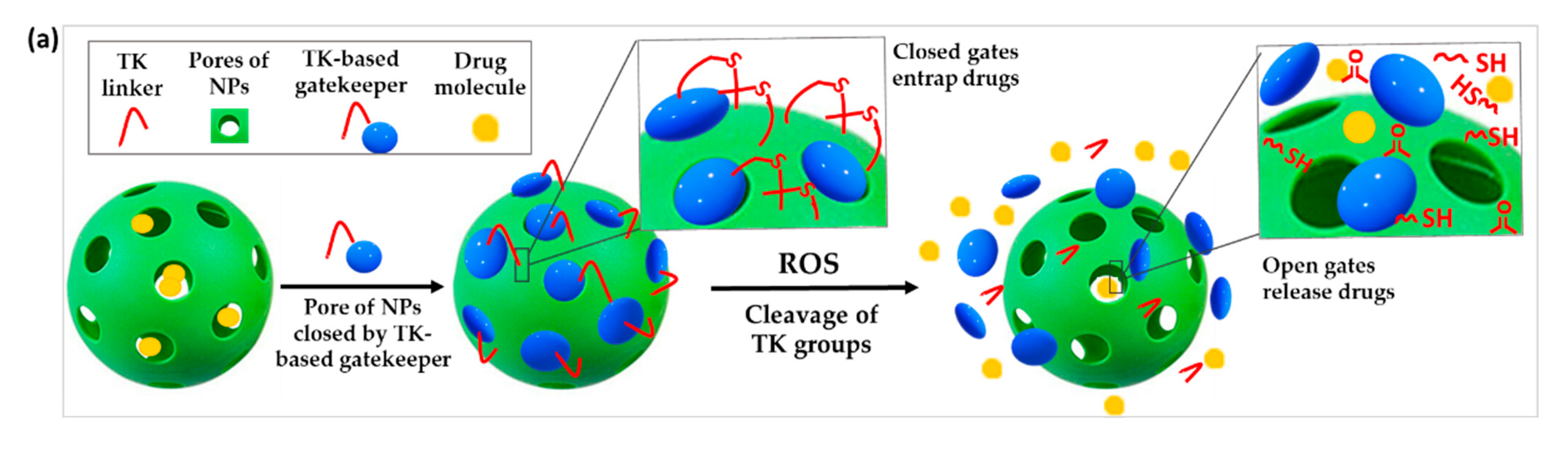
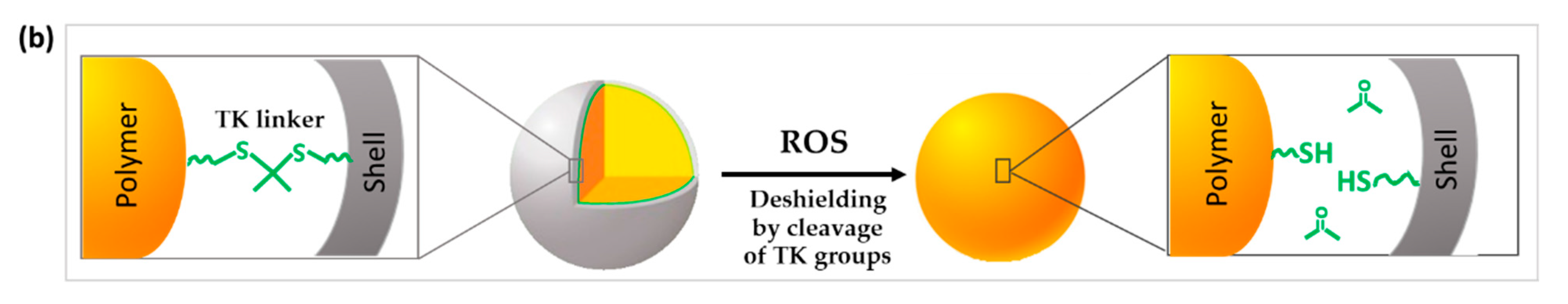
Figure 3. Schematic illustration of the chemical structure and corresponding release mechanisms of TK-based ROS-responsive nanosystems where TK is used to (a) create ROS-responsive drug-releasing gates or (b) induce the surface deshielding in response to ROS.
For instance, Shi et al. developed a novel type of mesoporous titanium dioxide NPs. After loading the anticancer drug docetaxel in the pores of the NPs, they were blocked by attaching β-cyclodextrin (β-CD) to the outer surface via a TK linker. The obtained NPs selectively released the drug only after ultrasound-induced ROS production which cleaved the ROS-sensitive linker and detached the gatekeepers [26]. Similar experiments were performed by Hu et al. using mesoporous silica nanoparticles (MSNs) for the co-encapsulation of DOX and the ROS producing agent α-tocopheryl succinate (α-TOS). These drugs were similarly blocked in the MSN pores by conjugating a TK-β-CD conjugate to the surface of the NPs. Less than 20% of DOX was leaked after 72 h of incubation in absence of ROS, proving the efficiency of the gating procedure. In contrast, 60% drug was released after a 72 h incubation with 100 μM H2O2. When dosed into human breast cancer (MCF-7) cells, increased intracellular ROS induced by the co-release of α-TOS further triggered the cleavage of the TK linker and release of DOX for enhanced chemotherapeutic effect [27].
3.2. TK Used to Change the Surface Characteristics or the Conformation of the DDS
As shown in the previous section, TK functional groups have been used to prepare DDS that degrade or release conjugated drugs from prodrugs or NPs upon ROS stimulation, however, TK moieties have also been exploited to design DDS that change surface characteristics or their conformation with the aim of improving cell-uptake and site-specific drug delivery in response to ROS.
In this context, Li et al. developed a nanosystem that changes the surface characteristics by controlling polyethylene glycol (PEG) shielding/deshielding at the desired site of action (Figure 3b). Paclitaxel (PTX) and chlorin e6 (Ce6) were co-encapsulated into NPs formed by the self-assembly of a diblock copolymer of PEG conjugated with poly(D,L-lactic acid) (PLA) via a TK bridge (PEG-TK-PLA NPs). While PEGylation of PEG-TK-PLA NPs prolongs their circulation time in blood, the exposure to high levels of ROS induced the breakage of the TK groups and the rapid loss of the PEG corona shell. This was was demonstrated by measuring the amount of released thiol-terminated PEG residues released after TK cleavage by Ellman’s reagent assay. In contrast, a negligible deshielding was observed in control PEG-PLA NPs. PEG deshielding did not affect the size or the morphology of the NPs but significantly enhanced cellular uptake, probably due to a lower surface PEG density [28]. In this study, enhanced levels of ROS sufficient to cleave the TK groups and achieve deshielding were produced after Ce6 activation triggered by the exposure to 600-nm light irradiation.
Regarding the application of TK to change the conformation of NPs, an example is provided by Cheng et al. They modified the side chains of polyvinyl alcohol (PVA) with cytotoxic mitochondrial targeted KLAK peptides and diblock co-polymers of a second peptide (KLVFF) conjugated to PEG by means of TK linker (KLVFF-TK-PEG). The modified PVA self-assembled into NPs, intended to specifically target mitochondria in cancer cells, while once inside the diseased cells the hydrophilic PEG chains were cleaved due to high levels of intracellular ROS. PEG detachment induced a morphology switch of the NPs into fibrous structures which exposed multiple KLAK molecules on the surface. KLAK then interacted with the mitochondria and provoked their disruption in cancer cells, ensuring high cytotoxicity in in vitro and in vivo models [29].
References
- Tao, W.; He, Z. ROS-Responsive Drug Delivery Systems for Biomedical Applications. Asian J. Pharm. Sci. 2018, 13, 101–112.
- Shi, J.; Wang, B.; Wang, L.; Lu, T.; Fu, Y.; Zhang, H.; Zhang, Z. Fullerene (C 60 )-Based Tumor-Targeting Nanoparticles with “off-on” State for Enhanced Treatment of Cancer. J. Control. Release 2016, 235, 245–258.
- Oddone, N.; Pederzoli, F.; Duskey, J.T.; De Benedictis, C.A.; Grabrucker, A.M.; Forni, F.; Angela Vandelli, M.; Ruozi, B.; Tosi, G. ROS-Responsive “Smart” Polymeric Conjugate: Synthesis, Characterization and Proof-of-Concept Study. Int. J. Pharm. 2019, 570, 118655.
- Redasani, V.K.; Bari, S.B. (Eds.) Chapter 2—Concept of Prodrug. In Prodrug Design; Academic Press: Cambridge, MA, USA, 2015; pp. 7–20. ISBN 978-0-12-803519-1.
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and Clinical Applications. Nat. Rev. Drug. Discov. 2008, 7, 255–270.
- Taresco, V.; Alexander, C.; Singh, N.; Pearce, A.K. Stimuli-Responsive Prodrug Chemistries for Drug Delivery. Adv. Ther. 2018, 1, 1800030.
- Oddone, N.; Boury, F.; Garcion, E.; Grabrucker, A.M.; Martinez, M.C.; Da Ros, F.; Janaszewska, A.; Forni, F.; Vandelli, M.A.; Tosi, G.; et al. Synthesis, Characterization, and In Vitro Studies of an Reactive Oxygen Species (ROS)-Responsive Methoxy Polyethylene Glycol-Thioketal-Melphalan Prodrug for Glioblastoma Treatment. Front. Pharmacol. 2020, 11, 574.
- Ryrfeldt, A.; Andersson, P.; Edsbäcker, S.; Tönnesson, M.; Davies, D.; Pauwels, R. Pharmacokinetics and Metabolism of Budesonide, a Selective Glucocorticoid. Eur. J. Respir. Dis. Suppl. 1982, 122, 86–95.
- Li, S.; Xie, A.; Li, H.; Zou, X.; Zhang, Q. A Self-Assembled, ROS-Responsive Janus-Prodrug for Targeted Therapy of Inflammatory Bowel Disease. J. Control. Release 2019, 316, 66–78.
- Swierczewska, M.; Lee, K.C.; Lee, S. What Is the Future of PEGylated Therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536.
- Pan, Q.; Deng, X.; Gao, W.; Chang, J.; Pu, Y.; He, B. ROS Triggered Cleavage of Thioketal Moiety to Dissociate Prodrug Nanoparticles for Chemotherapy. Colloids Surf. B Biointerfaces 2020, 194, 111223.
- Lin, C.; Tao, Y.; Saw, P.E.; Cao, M.; Huang, H.; Xu, X. A Polyprodrug-Based Nanoplatform for Cisplatin Prodrug Delivery and Combination Cancer Therapy. Chem. Commun. 2019, 55, 13987–13990.
- Li, M.; Jiang, S.; Haller, A.; Wirsching, S.; Fichter, M.; Simon, J.; Wagner, M.; Mailänder, V.; Gehring, S.; Crespy, D.; et al. Encapsulation of Polyprodrugs Enables an Efficient and Controlled Release of Dexamethasone. Nanoscale Horiz. 2021, 6, 791–800.
- Yang, K.; Yang, Z.; Yu, G.; Nie, Z.; Wang, R.; Chen, X. Polyprodrug Nanomedicines: An Emerging Paradigm for Cancer Therapy. Adv. Mater. 2021, 34, 2107434.
- Yin, W.; Ke, W.; Chen, W.; Xi, L.; Zhou, Q.; Mukerabigwi, J.F.; Ge, Z. Integrated Block Copolymer Prodrug Nanoparticles for Combination of Tumor Oxidative Stress Amplification and ROS-Responsive Drug Release. Biomaterials 2019, 195, 63–74.
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Liu, Y.; Ayyash, D.; Rasmussen, J.; Huo, M.; et al. ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy. Adv. Mater. 2017, 29, 1700141.
- Pei, P.; Sun, C.; Tao, W.; Li, J.; Yang, X.; Wang, J. ROS-Sensitive Thioketal-Linked Polyphosphoester-Doxorubicin Conjugate for Precise Phototriggered Locoregional Chemotherapy. Biomaterials 2019, 188, 74–82.
- Kim, J.S.; Jo, S.D.; Seah, G.L.; Kim, I.; Nam, Y.S. ROS-Induced Biodegradable Polythioketal Nanoparticles for Intracellular Delivery of Anti-Cancer Therapeutics. J. Ind. Eng. Chem. 2015, 21, 1137–1142.
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally Delivered Thioketal Nanoparticles Loaded with TNF-α–SiRNA Target Inflammation and Inhibit Gene Expression in the Intestines. Nat. Mater. 2010, 9, 923–928.
- Wang, G.-Y.; Jiang, H.; Yu, Y.; He, F.; Liu, Y.; Wang, Z.; Xiao, S.; Tang, C.; Xia, Z.; Ji, S.; et al. A New Method of Wound Treatment: Targeted Therapy of Skin Wounds with Reactive Oxygen Species-Responsive Nanoparticles Containing SDF-1α. Int. J. Nanomed. 2015, 10, 6571–6585.
- Li, Q.; Wen, Y.; Wen, J.; Zhang, Y.-P.; Xu, X.-D.; Victorious, A.; Zavitz, R.; Xu, X. A New Biosafe Reactive Oxygen Species (ROS)-Responsive Nanoplatform for Drug Delivery. RSC Adv. 2016, 6, 38984–38989.
- Li, Q.; Wen, Y.; You, X.; Zhang, F.; Shah, V.; Chen, X.; Tong, D.; Wei, X.; Yin, L.; Wu, J.; et al. Development of a Reactive Oxygen Species (ROS)-Responsive Nanoplatform for Targeted Oral Cancer Therapy. J. Mater. Chem. B 2016, 4, 4675–4682.
- Sun, C.; Liang, Y.; Hao, N.; Xu, L.; Cheng, F.; Su, T.; Cao, J.; Gao, W.; Pu, Y.; He, B. A ROS-Responsive Polymeric Micelle with a π-Conjugated Thioketal Moiety for Enhanced Drug Loading and Efficient Drug Delivery. Org. Biomol. Chem. 2017, 15, 9176–9185.
- Gisbert-Garzarán, M.; Vallet-Regí, M. Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 916.
- Vijayakameswara, R.N.; Han, H.S.; Lee, H.; Nguyen, V.Q.; Jeon, S.; Jung, D.-W.; Lee, J.; Yi, G.-R.; Park, J.H. ROS-Responsive Mesoporous Silica Nanoparticles for MR Imaging-Guided Photodynamically Maneuvered Chemotherapy. Nanoscale 2018, 10, 9616–9627.
- Shi, J.; Chen, Z.; Wang, B.; Wang, L.; Lu, T.; Zhang, Z. Reactive Oxygen Species-Manipulated Drug Release from a Smart Envelope-Type Mesoporous Titanium Nanovehicle for Tumor Sonodynamic-Chemotherapy. ACS Appl. Mater. Interfaces 2015, 7, 28554–28565.
- Hu, J.-J.; Lei, Q.; Peng, M.-Y.; Zheng, D.-W.; Chen, Y.-X.; Zhang, X.-Z. A Positive Feedback Strategy for Enhanced Chemotherapy Based on ROS-Triggered Self-Accelerating Drug Release Nanosystem. Biomaterials 2017, 128, 136–146.
- Li, J.; Sun, C.; Tao, W.; Cao, Z.; Qian, H.; Yang, X.; Wang, J. Photoinduced PEG Deshielding from ROS-Sensitive Linkage-Bridged Block Copolymer-Based Nanocarriers for on-Demand Drug Delivery. Biomaterials 2018, 170, 147–155.
- Cheng, D.-B.; Zhang, X.-H.; Gao, Y.-J.; Ji, L.; Hou, D.; Wang, Z.; Xu, W.; Qiao, Z.-Y.; Wang, H. Endogenous Reactive Oxygen Species-Triggered Morphology Transformation for Enhanced Cooperative Interaction with Mitochondria. J. Am. Chem. Soc. 2019, 141, 7235–7239.
More
