The Zika virus (ZIKV) was first isolated from a rhesus macaque in the Zika forest of Uganda in 1947. Isolated cases were reported until 2007, when the first major outbreaks of Zika infection were reported from the Island of Yap in Micronesia and from French Polynesia in 2013. In 2015, ZIKV started to circulate in Latin America, and in 2016, ZIKV was considered by WHO to be a Public Health Emergency of International Concern due to cases of Congenital Zika Syndrome (CZS), a ZIKV-associated complication never observed before. After a peak of cases in 2016, the infection incidence dropped dramatically but still causes concern because of the associated microcephaly cases, especially in regions where the dengue virus (DENV) is endemic and co-circulates with ZIKV. A vaccine could be an important tool to mitigate CZS in endemic countries. However, the immunological relationship between ZIKV and other flaviviruses, especially DENV, and the low numbers of ZIKV infections are potential challenges for developing and testing a vaccine against ZIKV.
- Zika
- dengue
- cross-reactivity
- vaccine
- pathogenesis
- CHIM
1. Introduction
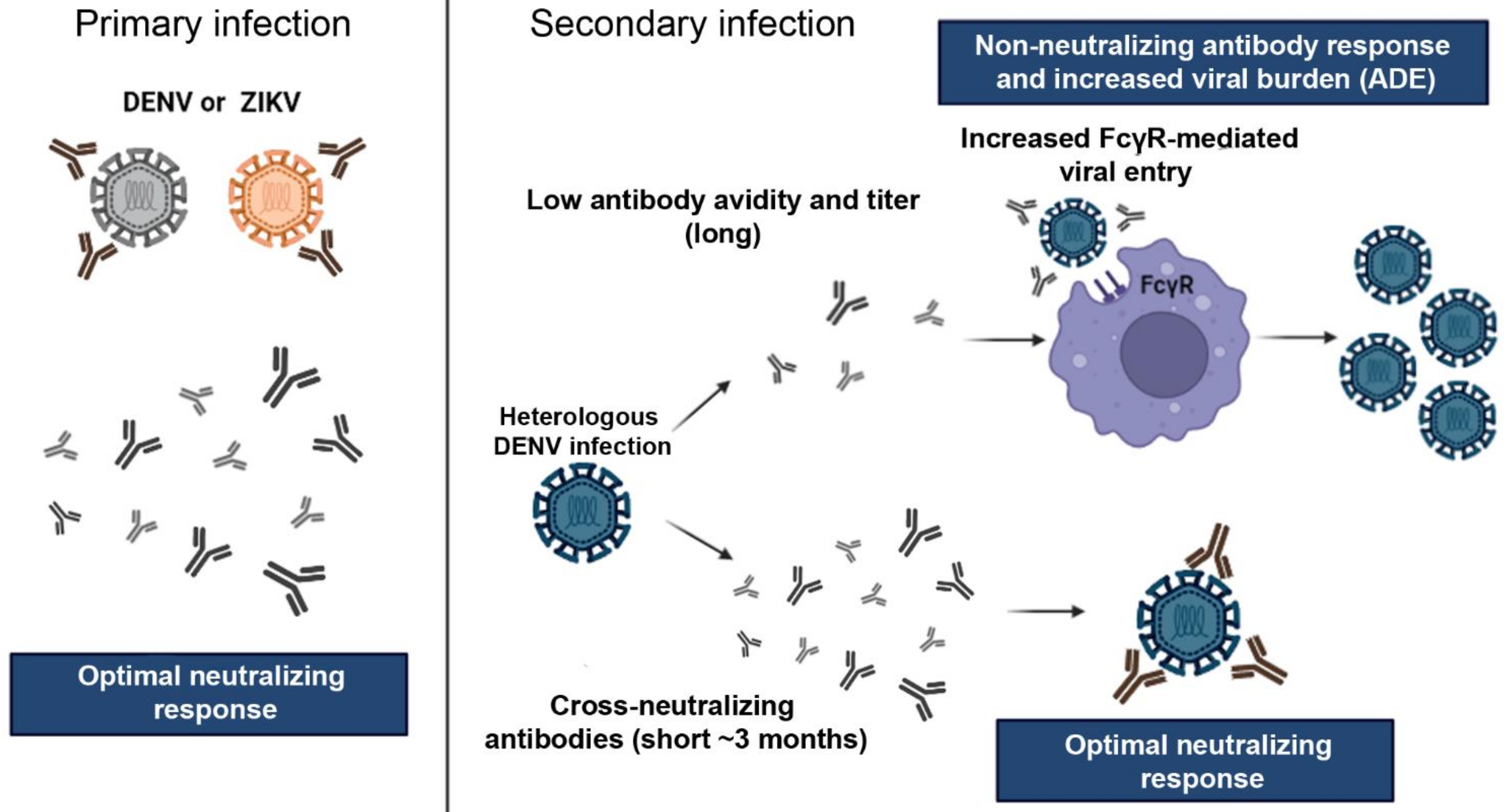
2. Implications of Flaviviruses Immunity on ZIKV Vaccine Development
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520.
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863.
- Desgraupes, S.; Hubert, M.; Gessain, A.; Ceccaldi, P.E.; Vidy, A. Mother-to-child transmission of Arboviruses during breastfeeding: From epidemiology to cellular mechanisms. Viruses 2021, 13, 1312.
- Gimenez-Richarte, A.; de Salazar, M.O.; Arbona, C.; Gimenez-Richarte, M.P.; Collado, M.; Fernandez, P.L.; Quiles, F.; Clavijo, C.; Marco, P.; Ramos-Rincon, J.M. Prevalence of chikungunya, dengue and Zika viruses in blood donors: A systematic literature review and meta-analysis. Blood Transfus. 2021; online ahead of print.
- Yuan, X.; Lou, Y.; He, D.; Wang, J.; Gao, D. A Zika endemic model for the contribution of multiple transmission routes. Bull. Math. Biol. 2021, 83, 111.
- Hayes, E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350.
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543.
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086.
- PAHO/WHO, PAHO. Timeline of Emergence of Zika Virus in the Americas. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=11959:timeline-of-emergence-of-zika-virus-in-the-americas&Itemid=41711&lang=en (accessed on 27 November 2021).
- Faria, N.R.; Azevedo, R.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349.
- Giovanetti, M.; Faria, N.R.; Nunes, M.R.T.; de Vasconcelos, J.M.; Lourenco, J.; Rodrigues, S.G.; Vianez, J.L., Jr.; da Silva, S.P.; Lemos, P.S.; Tavares, F.N.; et al. Zika virus complete genome from Salvador, Bahia, Brazil. Infect. Genet. Evol. 2016, 41, 142–145.
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 59–62.
- PAHO. Epidemiological Alert: Neurological Syndrome, Congenital Malformations, and Zika Virus Infection. Implications for Public Health in the America. 2015. Available online: https://iris.paho.org/handle/10665.2/50697?show=full (accessed on 20 December 2021).
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 47, 6–7.
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334.
- Calvet, G.; Aguiar, R.S.; Melo, A.S.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.; de Sequeira, P.C.; de Mendonca, M.C.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660.
- Cardenas, D.M.; Jaimes, M.A.; Vega, L.D.; Oliveros, N.L.; Soto, J.A.; Chia, C.R.; Osorio, J.E.; Ciuoderis, K.A. Immunological memory to Zika virus in a University Community in Colombia, South America. Acad. Bras. Cienc. 2020, 92, e20190883.
- Netto, E.M.; Moreira-Soto, A.; Pedroso, C.; Hoser, C.; Funk, S.; Kucharski, A.J.; Rockstroh, A.; Kummerer, B.M.; Sampaio, G.S.; Luz, E.; et al. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. mBio 2017, 8, e01390-17.
- Barreto, F.K.A.; Alencar, C.H.; Araujo, F.M.C.; Oliveira, R.; Cavalcante, J.W.; Lemos, D.R.Q.; Farias, L.; Boriz, I.L.F.; Medeiros, L.Q.; Melo, M.N.P.; et al. Seroprevalence, spatial dispersion and factors associated with flavivirus and chikungunha infection in a risk area: A population-based seroprevalence study in Brazil. BMC Infect. Dis. 2020, 20, 881.
- WHO. Zika Epidemiology Update. July 2019. Available online: https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1 (accessed on 20 December 2021).
- PAHO/WHO. Cases of Zika Virus Disease by Country or Territory. Weekly Report. 2021. Available online: https://www3.paho.org/data/index.php/en/?option=com_content&view=article&id=524:zika-weekly-en&Itemid=352 (accessed on 20 December 2021).
- Jacques, I.; Katz, L.; Sena, M.A.; Guimaraes, A.B.G.; Silva, Y.L.; Albuquerque, G.D.M.; Pereira, R.O.; de Albuquerque, C.; Silva, M.A.L.; Oliveira, P.A.S.; et al. High Incidence of Zika or Chikungunya Infection among pregnant women hospitalized due to obstetrical complications in Northeastern Brazil-Implications for laboratory screening in Arbovirus endemic area. Viruses 2021, 13, 744.
- Henein, S.; Swanstrom, J.; Byers, A.M.; Moser, J.M.; Shaik, S.F.; Bonaparte, M.; Jackson, N.; Guy, B.; Baric, R.; de Silva, A.M. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J. Infect. Dis. 2017, 215, 351–358.
- Dayan, G.H.; Galan-Herrera, J.F.; Forrat, R.; Zambrano, B.; Bouckenooghe, A.; Harenberg, A.; Guy, B.; Lang, J. Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naive adults in Mexico. Hum. Vaccines Immunother. 2014, 10, 2853–2863.
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012, 380, 1559–1567.
- Torresi, J.; Richmond, P.C.; Heron, L.G.; Qiao, M.; Marjason, J.; Starr-Spires, L.; van der Vliet, D.; Jin, J.; Wartel, T.A.; Bouckenooghe, A. Replication and excretion of the live attenuated tetravalent dengue vaccine CYD-TDV in a Flavivirus-naive adult population: Assessment of vaccine viremia and virus shedding. J. Infect. Dis. 2017, 216, 834–841.
- Harenberg, A.; Begue, S.; Mamessier, A.; Gimenez-Fourage, S.; Ching Seah, C.; Wei Liang, A.; Li Ng, J.; Yun Toh, X.; Archuleta, S.; Wilder-Smith, A.; et al. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum. Vaccines Immunother. 2013, 9, 2317–2325.
- Guirakhoo, F.; Pugachev, K.; Zhang, Z.; Myers, G.; Levenbook, I.; Draper, K.; Lang, J.; Ocran, S.; Mitchell, F.; Parsons, M.; et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 2004, 78, 4761–4775.
- Guirakhoo, F.; Weltzin, R.; Chambers, T.J.; Zhang, Z.X.; Soike, K.; Ratterree, M.; Arroyo, J.; Georgakopoulos, K.; Catalan, J.; Monath, T.P. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J. Virol. 2000, 74, 5477–5485.
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018, 379, 327–340.
- Villar, L.; Dayan, G.H.; Arredondo-Garcia, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramirez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123.
- Hadinegoro, S.R.; Arredondo-Garcia, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206.
- Moodie, Z.; Juraska, M.; Huang, Y.; Zhuang, Y.; Fong, Y.; Carpp, L.N.; Self, S.G.; Chambonneau, L.; Small, R.; Jackson, N.; et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J. Infect. Dis. 2018, 217, 742–753.
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54.
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M.; et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26, 5712–5721.
- Castanha, P.M.S.; Erdos, G.; Watkins, S.C.; Falo, L.D., Jr.; Marques, E.T.A.; Barratt-Boyes, S.M. Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI Insight 2020, 5, e133653.
- Fagbami, A.H.; Halstead, S.B.; Marchette, N.J.; Larsen, K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios 1987, 49, 49–55.
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128.
- Castanha, P.M.S.; Marques, E.T.A. Zika vaccines: Can we solve one problem without creating another one? Lancet Infect. Dis. 2021, 21, 1198–1200.
- Skibinski, D.A.; Baudner, B.C.; Singh, M.; O’Hagan, D.T. Combination vaccines. J. Glob. Infect. Dis. 2011, 3, 63–72.
- Nivarthi, U.K.; Swanstrom, J.; Delacruz, M.J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Kirkpatrick, B.D.; Pierce, K.K.; Diehl, S.A.; Katzelnick, L.; et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021, 12, 1102.
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silvera, C.G.; Costa, P.R.; et al. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J. Infect. Dis. 2015, 212, 1743–1751.
