Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Marco Campera.
Accelerometers offer unique opportunities to study the behaviour of cryptic animals but require validation to show their accuracy in identifying behaviours. This validation is often undertaken in captivity before use in the wild. While zoos provide important opportunities for trial field techniques, they must consider the welfare and health of the individuals in their care and researchers must opt for the least invasive techniques.
- animal training plan
- positive reinforcement
- Strepsirrhini
- animal welfare
1. Introduction
Remote measurement of animal behaviours has now been made possible through the availability of bio-logging devices such as accelerometers. The reduction in the size of microprocessors and increase in memory storage and battery life means that these devices can now be used on smaller species including arboreal species living in often inaccessible habitats [1]. For taxa that are cryptic, nocturnal, or elusive, traditional behavioural approaches may miss a considerable portion of their activity. Thus, data from accelerometers can reduce observer bias, quantify fine scale movements, and provide detailed data on the energetics of species [2]. At the same time, equipping cryptic taxa with accelerometers can prove challenging, involve multiple recaptures, and ensuring the longevity of devices in often harsh climates.
For these reasons, most accelerometer studies have been conducted on captive, domestic, or aquatic taxa. For arboreal species, the difficulties of catching and attaching loggers may be confounded by trial and error, where the loggers or collar styles fail [3,4][3][4]. Retrieving the loggers can place further constraints, and thus may result in low sample sizes in the wild, where the expense and risk of applying loggers must be considered [5]. Furthermore, for species that are difficult to observe continuously, validation in captivity is necessary to analyse the data collected by the loggers [6]. Finally, catching animals and putting a potentially hazardous device on them, a process that may require an equally risky anaesthesia, needs to be considered, especially for species that are globally threatened [4].
One of the key objectives of modern zoos is to aid in the in situ conservation of threatened species. Although this is frequently conducted through public education or the financial support of conservation projects, the potential to trial field techniques in captive animals occurs less often. Zoos, of course, must consider the welfare and health of the individuals in their care, with unnecessary animal handling practices being used as little as possible, and often only during the health checks of animals. At the same time, trialling field techniques such as the application of radio collars or accelerometers can be carried out in a safe setting with a veterinarian on hand. Scientists can then be equipped with the training to carry out such procedures on wild populations of endangered taxa. Furthermore, validation of accelerometers in captivity provides essential baseline data, allowing researchers to more quickly understand data collected in the wild [7].
Positive reinforcement training (PRT) is a form of operant conditioning learning increasingly used by zoos, which involves rewarding animals to elicit specific behaviours [8]. By associating behaviours important for veterinary, husbandry, or scientific procedures with a positive experience (i.e., rewards), the animal can gain motivation to engage in the behaviour [9]. PRT may start with food rewards, but by linking these rewards with an alternative action or associated words, the behaviour can be stimulated by such actions alone. Target training is an increasingly commonly used PRT strategy, whereby an animal is trained to touch or be touched by an object such as a dowel or plastic target stick [10]. These training sessions, for example, are meant to help with the delivery of health checks and reduce the incidence of undesirable behaviours. In addition to the training goal, target training can be an important additional element to enrichment and to increasing the relationship between an animal and a keeper (e.g., by reducing potential risks of bites or scratches for keepers) [11].
Slow lorises (Nycticebus spp.) are globally threatened nocturnal primates, nine species of which are found in Southeast Asia, but which are scarce in zoos due to poor neonate survivorship [12]. In the wild, they are frequently found in dense tangles and bamboo thickets, meaning that they are out of view of the researchers for about 30% of the time [13,14][13][14]. Their locomotion is evolutionarily unique amongst primates in that they do not leap, and along with their unique slow energetics, their activity patterns and locomotion have long been of interest to researchers [15]. Most captive studies, however, usually record locomotion and energetics on a single substrate that is vastly different to their complex habitat use in the wild [16]. Wild slow lorises readily wear radio collars and tend not to remove them [17,18][17][18]. Activity score accelerometers have been successfully applied to wild Javan slow lorises (N. javanicus). These accelerometers yield less specific information on activity but have been useful in sleep research [5]. Three-axis accelerometers, however, can provide more information on these cryptic species, for example, lorises are out of sight of direct observation around 30–40% of the time [14], meaning that wresearchers are missing a large proportion of behaviours.
2. Training for Application of the Device
In order to train Tina to become accustomed to having the collar placed over her head and around her neck, wresearchers presented the target stick as a focus on the other side of the collar. Before introducing the cat collar, wethey initially introduced a larger bamboo leaf in the shape of a collar that was larger than the diameter of her head, so she could move in and out freely. WeResearchers held the target stick in the centre of the collar to encourage Tina to move her head towards it; once she touched the target with her nose or was close, wethey gently held the collar over her head for a few seconds (Figure 1).
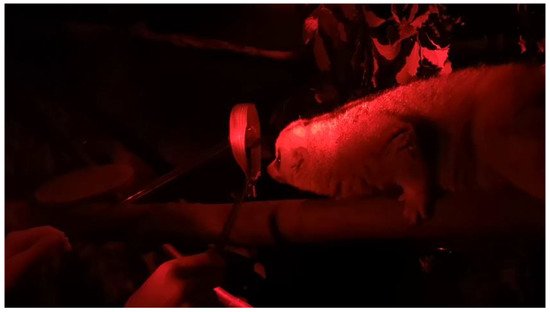

Figure 1. Tina the Bengal slow loris being presented with the bamboo leaf collar with tongs during the initial stage of the training.
At this point, wresearchers began to touch Tina’s neck with the cat collar. This stage took several attempts as Tina needed to adjust to the new collar. Any time she regressed, wethey moved a step back and rewarded her for just touching the target stick to keep her engaged. After 12 sessions, she began to push her head through the collar to touch the target stick.
This allowed Tina to choose to touch the collar herself by moving her head through the loop to touch the target stick on the other side. The target stick was slowly moved further out of the loop of the collar away from Tina on each session so that she had to reach further through the collar. WResearchers then progressed to placing the collar directly over Tina’s head using the tongs once she had pushed her head through to touch the target stick. The collar was adjusted so that it was relatively loose to allow us to wiggle the collar from side to side to fit over her neck. After only two sessions, wethey could place the collar fully over her head (Figure 2).
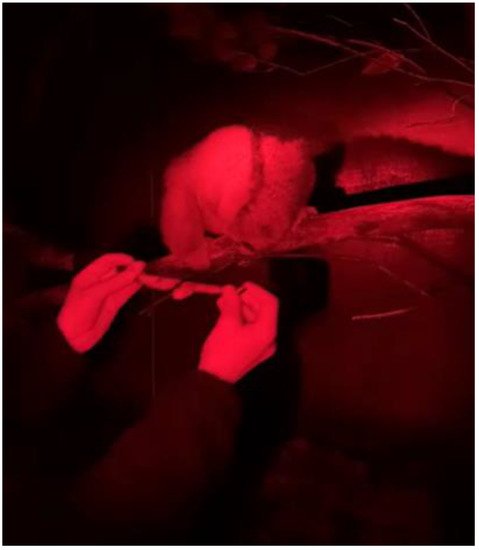

Figure 2. Tina the Bengal slow loris at the final stage of the training when wresearchers were able to safely cat collar and equip her with the accelerometer device with outheir hands.
Slow lorises can bite and are also venomous, so wresearchers deemed it prudent also to remove the collar with tongs. To achieve this, wethey used the words “Tina, touch” as a prompt that something was about to happen, and then to introduce a new command. WeResearchers used the tongs to manipulate the collar whilst it was on her head, after which wethey said “touch”, and gently moved the collar around on her neck. She allowed this in the first session, but to reinforce the behaviour, weresearchers added three additional sessions. After this, wethey added the accelerometer to allow Tina to acclimatise to the smell and weight. As reinforcement, weresearchers allowed her to eat her preferred insects whilst wearing the collar for a few minutes at a time.
For ease of fitting and removing the collar, wresearchers next trained Tina to allow this to be undertaken by hand. This training also distinguished the collar work from general feeding, when keepers always wear gloves. Saying the touch command, wethey started to replace the tongs with the keeper’s left hand, held up flat vertically facing Tina’s face with the palm forward. WeResearchers followed this by gently placing the right hand over the back of her neck and moving the collar. She was wary at first, so wethey introduced this keeper touch command without the collar on intermittent days to the collar training. This involved using the target command to start the session to reinforce her, followed by using the flat hand command, saying “Tina, touch”, and touching the back of her neck with the right hand, applying very gentle pressure each time. She was rewarded with the term “good” if she sat still during this process. It took her 12 sessions to acclimatise to being touched, with each session being slightly longer and touching her neck more to mimic wearing the collar. To start her becoming used to wearing the actual collar, weresearchers held the collar flat in front of her and gently touching underneath her neck and using the “touch” command. After two sessions, she allowed us to mimic clipping the collar around her neck. With the success of the training, Tina wore the collar during her active period. Despite biting at the accelerometer three times after it was initially placed on her, with each bout of biting decreasing in severity and longevity, she quickly grew used to it and allowed us to remove it during her inactive periods on a regular basis. The collar was removed only when the animal was conscious and had approached a zookeeper to minimise the level of disturbance.
3. Accelerometer Validation
The variables associated with the accelerometers had an accuracy of 80.7 ± SD 9.9% in predicting the ten behaviours of the captive Bengal slow loris (Table 1). The highest accuracy was obtained with resting behaviour (99.8%), while the lowest accuracy was obtained for suspensory walk (60.3%), which was mainly confused by the algorithm with suspensory feeding (18.8% of suspensory walk from the validation set was associated with suspensory feeding). ODBA was the most important variable to predict behaviours based on the mean decrease accuracy (i.e., estimate of the loss in prediction performance when a variable is omitted from the training set; Figure 3), while static lateral acceleration was the most important classifier (Figure 4) and had the highest mean decrease GINI (i.e., node impurity, so the higher it is, the more important the variable is to split the data correctly). Furthermore, static lateral acceleration was the most important classifier for seven behaviours out of ten. The only behaviour that was poorly classified by the static lateral acceleration was resting, where the amplitude of the lateral dynamic acceleration was more important. The body pitch was an important predictor for specific behaviours such as resting, bridge, walk, and feeding non-suspensory. Dynamic accelerations had the lowest importance as classifiers.
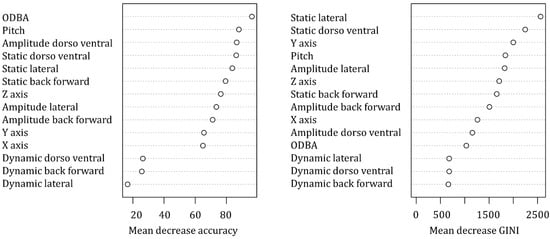
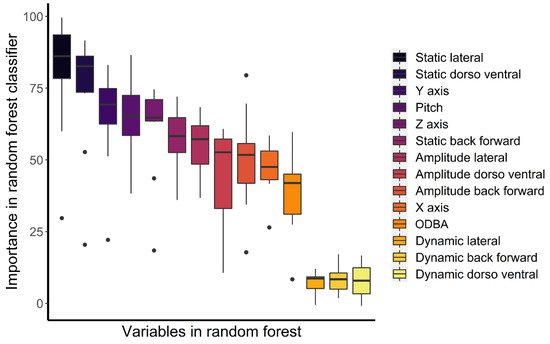

Figure 3. Mean decrease accuracy and mean decrease GINI of the predictor variables included in the random forest classifier.

Figure 4. Box plots of the importance as a classifier of the variables included in the random forest. Values are medians, quartiles, and ranges considering the ten behaviours tested. Points are outliers.
Table 1. Results of the random forest classification to assess the predictive power of the variables retrieved from a three-axis accelerometer in assessing the behaviours of a captive Bengal slow loris. Prediction accuracy and main confusing behaviour were based on the performance of the random forest model obtained from the training set of data in predicting the behaviours in the validation set. The importance in random forest classifier was based on the training set.
| Behaviour | Prediction Accuracy (%) | Main Confusing Behaviour (% error) | Importance in Random Forest Classifier | ||
|---|---|---|---|---|---|
| 1st Variable | 2nd Variable | 3rd Variable | |||
| Resting | 99.8 | Suspensory walk (0.2) | Amplitude lateral | Pitch | Static back forward |
| Bridge | 85.9 | Suspensory walk (4.3) | Pitch | Static lateral | Static dorso ventral |
| Suspensory feeding | 85.6 | Suspensory walk (5.1) | Static lateral | Static dorso ventral | Y axis |
| Climb down | 82.5 | Walk (8.7) | Static lateral | Static dorso ventral | Y axis |
| Climb up | 80.7 | Walk (9.6) | Static lateral | Static dorso ventral | Y axis |
| Alert | 80.4 | Walk (9.3) | Static lateral | Static dorso ventral | Amplitude back forward |
| Climb horizontally | 79.8 | Suspensory walk (15.5) | Static lateral | Static dorso ventral | Amplitude back forward |
| Walk | 77.2 | Alert (9.6) | Static lateral | Pitch | Static dorso ventral |
| Feeding non-suspensory | 75.0 | Suspensory feeding (9.0) | Static lateral | Static dorso ventral | Pitch |
| Suspensory walk | 60.3 | Feeding suspension (18.8) | Static lateral | Static dorso ventral | Z axis |
References
- Brown, D.D.; Kays, R.; Wikelski, M.; Wilson, R.; Klimley, A. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelem. 2013, 1, 20.
- Halsey, L.G.; Shepard, E.L.C.; Wilson, R.P. Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp. Biochem. Physiol. A 2011, 158, 305–314.
- Furnell, S. A Study of the Locomotor Ecology of the Indriid Primate Propithecus verreauxi in the Dry Deciduous Forest of Kirindy Mitea National Park, Madagascar. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2013.
- Campera, M.; Balestri, M.; Chimienti, M.; Nijman, V.; Nekaris, K.A.I.; Donati, G. Temporal niche separation between the two ecologically similar nocturnal primates Avahi meridionalis and Lepilemur fleuretae. Behav. Ecol. Sociobiol. 2019, 73, 55.
- Reinhardt, K.D.; Vyazovskiy, V.V.; Hernandez-Aguilar, R.A.; Imron, M.A.; Nekaris, K.A.I. Environment shapes sleep patterns in a wild nocturnal primate. Sci. Rep. 2019, 9, 9939.
- Ladds, M.A.; Salton, M.; Hocking, D.P.; McIntosh, R.R.; Thompson, A.P.; Slip, D.J.; Harcourt, R.G. Using accelerometers to develop time-energy budgets of wild fur seals from captive surrogates. PeerJ 2018, 6, e5814.
- Wunderlich, R.E.; Tongen, A.; Gardiner, J.; Miller, C.E.; Schmitt, D. Dynamics of locomotor transitions from arboreal to terrestrial substrates in Verreaux’s sifaka (Propithecus verreauxi). Integr. Comp. Biol. 2014, 54, 1148–1158.
- Skinner, B.F. Operant behavior. In Operant Behavior: Areas of Research and Application; Honig, W.K., Ed.; Appleton-Century-Crofts: New York, NY, USA, 1966; pp. 12–32.
- Prescott, M.J.; Buchanan-Smith, H.M. Training nonhuman primates using positive reinforcement techniques. J. Appl. Anim. Welf. Sci. 2003, 6, 157–161.
- Laule, G.E.; Bloomsmith, M.A.; Schapiro, S.J. The use of positive reinforcement training techniques to enhance the care management, and welfare of primates in the laboratory. J. Appl. Anim. Welf. Sci. 2003, 6, 163–173.
- Melfi, V. Is training zoo animals enriching? Appl. Anim. Behav. Sci. 2013, 147, 299–305.
- Fuller, G.; Lukas, K.E.; Kuhar, C.; Dennis, P.M. A retrospective review of mortality in lorises and pottos in North American zoos, 1980–2010. Endanger. Species Res. 2014, 23, 205–217.
- Rode-Margono, E.J.; Nijman, V.; Wirdateti, W.; Nekaris, K.A.I. Ethology of the Critically Endangered Javan slow loris Nycticebus javanicus E. Geoffroy Saint-Hilaire in West Java. Asian Primates 2014, 4, 27–41.
- Poindexter, S.; Nekaris, K.A.I. Vertical clingers and gougers: Rapid acquisition of adult limb proportions facilitates feeding behaviours in young Javan slow lorises (Nycticebus javanicus). Mamm. Biol. 2017, 87, 40–49.
- Hanna, J.B.; Schmitt, D. Locomotor energetics in primates: Gait mechanics and their relationship to the energetics of vertical and horizontal locomotion. Am. J. Phys. Anthropol. 2011, 145, 43–54.
- Stevens, N.J. The effect of branch diameter on primate gait sequence pattern. Am. J. Primatol. 2008, 70, 356–362.
- Wiens, F.; Zitzmann, A. Social structure of the solitary slow loris Nycticebus coucang (Lorisidae). J. Zool. 2003, 261, 35–46.
- Nekaris, K.A.I.; Campera, M.; Nijman, V.; Birot, H.; Rode-Margono, E.J.; Fry, B.G.; Imron, M.A. Slow lorises use venom as a weapon in intraspecific competition. Curr. Biol. 2020, 30, R1252–R1253.
More
