Besides the well-known antibacterial effect of lactoferrin, novel interest has been rising towards its potential application in the field of dry eye and viral infections. A growing body of evidence supports the antimicrobial efficacy of lactoferrin, which is not limited to its iron-chelating properties but also depends on its capability to directly interact with pathogen particles while playing immunomodulatory effects. Nowadays, lactoferrin antiviral activity is of special interest, since lactoferrin-based eye drops could be adopted to treat/prevent the new severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection, which has conjunctivitis among its possible clinical manifestations. In the future, further data from randomized controlled studies are desirable to confirm the efficacy of lactoferrin in the wide range of ocular conditions where it can be used.
- Sars-CoV-2
- antimicrobial peptides
- biofilm
- dry eye
- lactoferrin
- ocular surface
- viral infections.
Definition:Lactoferrin is a naturally occurring iron-binding glycoprotein, produced and secreted by mucosal epithelial cells and neutrophils in various mammalian species, including humans. It is typically found in fluids like saliva, milk and tears, where it reaches the maximum concentration. Thanks to its unique anti-inflammatory, antioxidant and antimicrobial activities, topical application of lactoferrin plays a crucial role in the maintenance of a healthy ocular surface system.
1. Introduction
The ocular surface system is an essential component of vision. It includes the cornea, conjunctiva, tear film, eyelids and lacrimal and meibomian glands, all components linked functionally by the continuity of the epithelia, innervation and endocrine, vascular and immune systems [1]. As a part of a complex morphofunctional unit, each constituent of this system acts to provide, protect and maintain a smooth refractive surface on the cornea, whose avascularity and transparency enable light to proceed through the lens onto the retina. As a direct interface between the eye and the external environment, the ocular surface is constantly exposed to potentially harmful agents, like microbes and toxic substances, which place it at risk of destructive immunological reactions. Hence, loss of the outer eye’s structural integrity may follow, leading to various degrees of visual impairment.
Host defenses at the level of the ocular surface include a combination of mechanical, anatomical and immunological mechanisms. Both conjunctival and corneal cells provide an epithelial barrier to prevent pathogen entrance, and concomitantly secrete cytokines to activate immune defenses against microbial invasion. Besides mediating reflexive blinking movements, corneal nerves play a key role in the maintenance of corneal trophism, and thanks to neuropeptide release they have the capability to induce cytokine activity and the subsequent neutrophil inflow [2][3][4][2,3,4]. Cooperation between innate and acquired arms of the immune system is crucial to keep the homeostatic balance of the ocular surface united once pathogen assault occurs, since the structural integrity of the whole system is required to ensure its normal functioning. Among the effectors of innate immunity, complement comprises a series of bioactive compounds such as enzymes, opsonins, anaphylatoxins and chemotoxins that are activated by means of a chain reaction and contribute to the onset of corneal inflammation. In addition, neutrophils and macrophages synergistically act to protect ocular surface epithelia from pathogen invasion. The former play a key role in phagocytosis and microbial killing, while the latter feature both phagocytic and antigen-presenting capabilities and adjuvate inflammatory reactions by secreting cytokines [5][6][5,6]. On the other hand, interferons (IFNs) and natural killer (NK) cells activate each other in response to viral infection. In particular, IFNs stimulate the production of major histocompatibility complex (MHC) class I molecules and proteins, which allow virally infected cells to be recognized by T cells, whereas IFN-mediated activation of NK lymphocytes leads to the targeting and subsequent elimination of virus-hosting cells [7]. Not least, tears also act as a source of unspecific antimicrobial substances like lysozyme, lactoferrin, lipocalins and secretory phospholipase A2, which are effective in counteracting the invasion and colonization of microorganisms at the ocular surface [8].
Although several antimicrobial drugs may be used to treat infections, concerns are rising in regard to the increasing prevalence of drug-resistant microbes (in particular for antibiotics) and the limited efficacy of the available compounds. Thus, it is important to look for and to develop additional drugs that could either overcome the issue of drug resistance, or to act synergistically when combined with other treatments. Antimicrobial peptides that are constitutively represented in human fluids and tissues may provide the basis for the development of new therapeutic agents to be successfully applied in the management of ocular surface infections.
2. Lactoferrin and Dry Eye
Topical application of lactoferrin has been shown to reduce irradiation-induced corneal epithelial damage in mice models, as well as to promote corneal wound healing after alkali-burn injury [9][10][16,17]. Furthermore, previous studies have reported a significant correlation between low levels of tear lactoferrin and the development of both dry eye disease (DED) and chronic meibomitis [11][12][13][18,19,20] (Figure 1Figure 2A). These two common disorders of the ocular surface system share a degree of mutual pathophysiology, since markers of inflammation and oxidative stress are present in both conditions [14][21]. Indeed, a decreased volume and an altered qualitative composition of tear film, together with excessive tear evaporation, lead to the creation of a hyperosmolar environment, which then initiates both inflammatory and oxidative cascades, resulting in impaired epithelial proliferation and differentiation [15][22]. The rationale of the use of lactoferrin in the setting of DED derives from its capacity to directly address the vicious cycle of the disease, especially the underlying inflammation and oxidative stress. In particular, thanks to its iron-chelating ability, lactoferrin provides oxygen free radical and hydroxyl scavenging activities, thus inhibiting pro-inflammatory and tissue damaging effects of reactive oxygen species (ROS). On the other hand, lactoferrin attenuates excessive inflammation in host responses to pathogens by inhibiting classical complement activation and by downregulating inflammatory mediators such as tumor necrosis factor (TNF)-alpha, interleukins (ILs)-1, -6 and -8, the intercellular adhesion molecule (ICAM)-1 and CD14 [23] [16](Figure 1). In a study by Dogru et al., patients supplemented with oral lactoferrin showed ameliorated dry eye symptoms and tear film stability [17][24]; another study reported its efficacy in improving ocular surface parameters, such as tear break-up time and the Schirmer test, in patients affected by dry eye induced by cataract surgery [18][25]. Furthermore, locally applied lactoferrin was able to restore corneal epithelial integrity in a rabbit model of dry eye, suggesting the potential use of lactoferrin eye drops for treating DED [19][26].
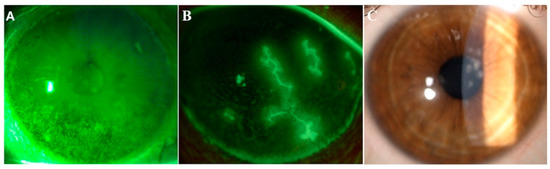
Figure 12. Images from three representative patients affected by dry eye, herpetic keratitis and adenoviral keratoconjunctivitis. Representative images of ocular conditions that may benefit from the use of lactoferrin: dry eye (A), herpetic keratitis (B) and epidemic keratoconjunctivitis (C). (a) Slit lamp photograph of the cornea of a patient with dry eye after the instillation of 20 μL of unpreserved 2% sodium fluorescein and use of the yellow filter to enhance the staining details. The epithelial damage is visible with fluorescein staining as multiple punctate epithelial erosions scattered over the corneal surface, in particular in the lower sectors. (b) Slit lamp photograph of the cornea of a patient with herpetic keratitis after the instillation of 20 μL of unpreserved 2% sodium fluorescein and use of the yellow filter to enhance the staining details. Classical dendritic epithelial defects are visible with positive fluorescein staining. (c) Slit lamp photograph of a patient with adenoviral conjunctivitis showing multifocal sub-epithelial (stromal) corneal infiltrates.
3. Conclusions
Nature provides us with several examples of the use of micronutrients with a medical purpose. As physiological constituents of human tissues, substances deriving from either food intake or nutraceutical products can act as bioactive compounds and influence both the morphology and function of the ocular surface system components by taking part in several metabolic cellular pathways aiming at preserving a homeostatic balance. In this regard, topical lactoferrin answers the need to develop new treatments to target infections at the ocular surface. This glycoprotein has some important advantages. First of all, it is easily found in nature and exhibits multiple beneficial effects on ocular tissues. Its efficacy as an antimicrobial agent has been widely reported, and interest regarding the application of lactoferrin in the setting of viral infections is growing. In this field, lactoferrin could be adopted either as a single therapeutic agent or in combination with other treatments to maximize efficacy. Further data from randomized controlled studies are desirable to confirm the efficacy of lactoferrin in the wide range of ocular conditions where it can be used.
