You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Charles L Leonard Greenblatt.
BCG vaccine has been used for 100 years to prevent tuberculosis. Not all countries, including the United States, adopted the initial World Health Organization recommendation to use BCG. Moreover, many Western countries that had routinely used BCG have discontinued its use.
- Bacillus Calmette–Guérin
- BCG
- plasma amyloid
- Alzheimer’s
- amyloid probability score (APS)
1. Introduction
The Bacillus Calmette–Guérin (BCG) vaccine has not only been viewed as an important intervention in tuberculosis control but as an adjuvant for other antigens and as a “pro-inflammatory” agent in cancer therapy. Very shortly after its introduction in humans, Calmette noted its effect, not just on the incidence of tuberculosis, but also on the general mortality of infants [1]. During the writing of this manuscript, in Lille France a conference was held to celebrate the 100th anniversary of BCG [2]. At this meeting, papers presented can be seen as a reminder of the amazing breadth of action of this attenuated bacterium in human disease and conditions. Childhood application was emphasized and a wide range of immunization methods were given. BCG not only appeared as its original self, but as a recombinant organism ready to do more and do it better. Special note was on allergies, and inflammatory and autoimmune diseases. Of special importance to outheir research, a paper at the conference cited its benefit in the reduction of the risk to developing Alzheimer’s Disease (AD) in BCG recipients with superficial bladder cancer [3]. The work in cancer patients was suggested by the lower levels of dementia in countries with universal BCG coverage [4]. In animal models, it was of great interest to us that BCG “reversed the cognitive decline” [5]. However, it should be noted that the mouse brain amyloid was not affected. In the human studies, instillation of BCG into the bladder would require a rethinking of this approach for the prevention of AD as a public health measure. The work presented here is an attempt to determine if two intradermal injections of BCG would affect the blood levels of Aβ42 and Aβ40.
Increasingly understood is that the pathology of AD predates clinical symptoms by decades [6,7][6][7]. Accurate blood-based tests for brain Aβ pathology are urgently needed to identify at-risk individuals and give the opportunity for disease modifying intervention in the preclinical stages of AD. A method has emerged that assesses plasma levels of Aβ42 and Aβ40. This method, from the Bateman laboratory at Washington University, immunoprecipitates Aβ to isolate it from plasma; this is followed by liquid chromatography–mass spectrometry (LC-MS) to determine Aβ42 and Aβ40 concentrations [8]. Aβ42 is lower in CSF and blood as this “stickier” form of the protein builds up in the plaques. Aβ40, being more hydrophilic, is used as the denominator in a ratio with Aβ42 to normalize inter-individual differences in Aβ production [9]. Thus, a lower Aβ42/Aβ40 ratio is considered to correlate with a greater risk of cerebral Aβ deposition.
2. Demographics, CMV Antibody Titer, and ApoE4 Allele
The study cohort consisted of 49 participants, of which 28 (57.1%) were female and 21 (42.9%) were males (Table 1). The average age of males was 65.7 years and was not significantly different (p = 0.52) from that of female participants (64.3 years). Similarly, the length of education of participants was not statistically significant between males and females (p = 0.47). The proportion of patients who tested positive for CMV, defined as IgG titer greater than 0.6 U/mL, was not significantly different between females and males (32.1% vs. 28.6%, p = 1). Although the proportion of male participants who had at least one ApoE4 allele was higher than that of female participants, the difference was not statistically significant (57.1% vs. 35.7%, p = 0.23). The SAGE scores were not significantly different between females and males either before or after BCG vaccine administration.
Table 1. Participant Demographics.
| Females | Males | p-Value | |
|---|---|---|---|
| n(%) | 28 (57.1) | 21 (42.9) | |
| Age, years | |||
| Mean (SD) | 64.3 (6.7) | 65.7 (8.4) | 0.52 a |
| Length of education, years | |||
| Median (IQR) | 16 (14–18) | 16 (16–18) | 0.47 b |
| CMV infection, n (%) | |||
| Negative | 19 (67.9%) | 15 (71.4%) | 0.79 c |
| Positive | 9 (32.1%) | 6 (28.6%) | |
| ApoE4 allele, n (%) | |||
| Negative | 18 (64.3%) | 9 (42.9%) | 0.14 c |
| Positive | 10 (35.7%) | 12 (57.1%) | |
| SAGE | |||
| Before BCG | |||
| Median (IQR) | 22 (21–22) | 22 (21.5–22) | 0.70 b |
| After BCG | |||
| Median (IQR) | 22 (22–22) | 22 (21–22) | 0.37 b |
a Two-sample independent t-test, b Mann–Whitney test, c Chi-square test.
3. BCG Significantly Increased the Aβ42/40 Ratio
The Aβ42/40 ratio for each participant was stratified by ApoE genotype (Figure 1). As can be seen, participants having at least one ApoE4 allele tended to have lower Aβ42/40 ratio compared to participants having other alleles. Figure 2 depicts a dot plot for individual values of amyloid 42/40 ratio in participants stratified by ApoE genotype. The figure shows that participants tended to have higher values of Aβ42/Aβ40 ratio after BCG vaccine administration compared to the baseline ratio before BCG vaccine administration regardless of ApoE4 allele status.
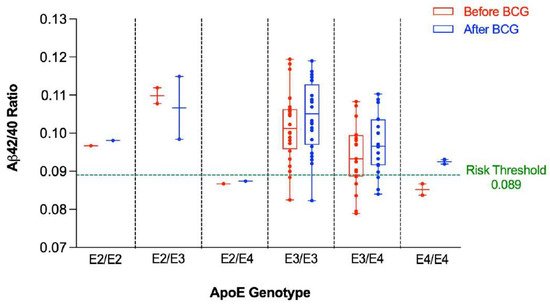
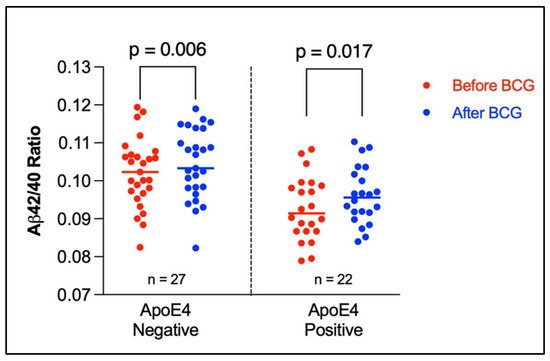

Figure 1. Aβ42/40 ratio before and after BCG vaccination stratified by ApoE genotype. An Aβ42/40 ratio below 0.089 is associated with higher risk of positive amyloid PET scan.

Figure 2. Amyloid 42/40 ratio before and after BCG vaccine administration stratified by ApoE4 alleles. The plot shows the individual values with median (horizontal segment). The number (n) denotes the number of observations. Statistical analysis was completed using Wilcoxon–Pratt Signed-Rank Test.
4. Younger Participants Had More of a “Favorable” Increase in Their Aβ42/40 Ratio Than Older Participants
When the Aβ42/40 ratio before and after BCG administration was stratified by age, participants ≤ 65 years old had a significant increase in the Aβ42/40 ratio post BCG vaccine compared to the baseline ratio before vaccine administration (0.10417 ± 0.0092 vs. 0.10006 ± 0.00901, p < 0.0001) (Figure 3). On the other hand, there was a non-significant increase in the Aβ42/40 ratio after BCG vaccination in older individuals > 65 years old (0.09628 ± 0.00785 vs. 0.09528 ± 0.01021, p = 0.49).
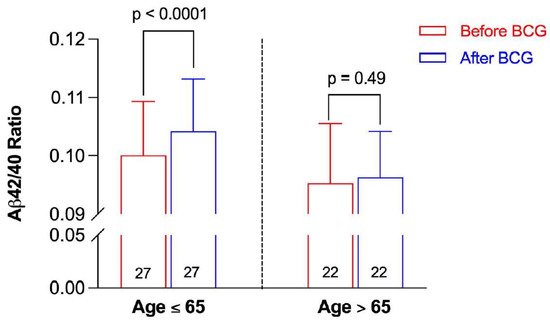

Figure 3. Aβ42/40 ratio before and after BCG vaccination stratified by age group. Data are presented as mean ± SD. The numbers inside the bars represent the number of observations. Statistical analysis was completed using a paired t-test.
5. High Risk Participants Had More of a “Favorable” Decrease in Their APS Than Intermediate Risk and Low Risk Participants
The change in APS following BCG vaccination in the participants was stratified by the risk of positive PET scan as assessed by the baseline APS. The APS was reduced after BCG administration in the three risk categories (Figure 4). However, such a reduction reached statistical significance only in the high-risk category (p = 0.016) (Figure 4).
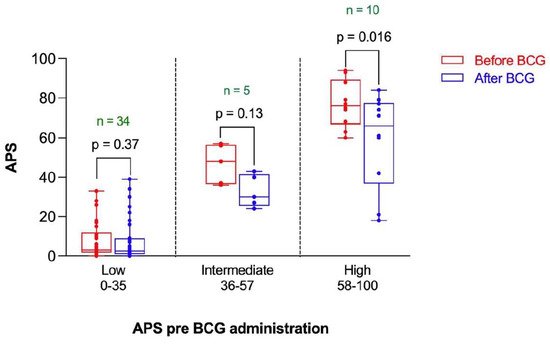

Figure 4. Amyloid Probability Score (APS) before and after BCG vaccination stratified by risk category based on APS pre-BCG administration. n represents the number of observations in each category. Statistical analysis was completed using a Wilcoxon–Pratt Signed-Rank Test.
6. Participants without Latent CMV Infection Had a “Favorable” Decrease in Their APS with BCG Compared to Participants Latently Infected with CMV
CMV is part of the herpes family of viruses, its designation HHV-5. CMV is mostly known as an opportunist causing life-threatening infection in HIV infected persons or in immunosuppressed organ transplant recipients. In immune competent individuals, infection with CMV is usually asymptomatic; once established, its containment becomes a priority for the immune system, which is unable completely to eliminate it and its continued presence promotes age-like immune changes [11,12][10][11]. The persistent presence of CMV diverts an extraordinary amount of the T-cell resource to keep this virus in check and in doing so results in a high immune risk profile (IRP) inverting the normal CD4/CD8 ratio (normally about 2:1). CMV has been implicated as a causal agent in AD [13,14][12][13]. While another study suggests that CMV carriage facilitates HSV1-associated AD [15][14].
Thus, the change in APS following BCG vaccination was stratified by the CMV IgG status. There was no significant difference in APS before BCG administration between participants having negative or positive CMV IgG (Figure 5). However, the APS was significantly reduced after BCG vaccination in participants having negative CMV IgG (p = 0.03), whereas there was no significant change in the APS following BCG vaccination in participants with positive CMV IgG (p = 0.13).
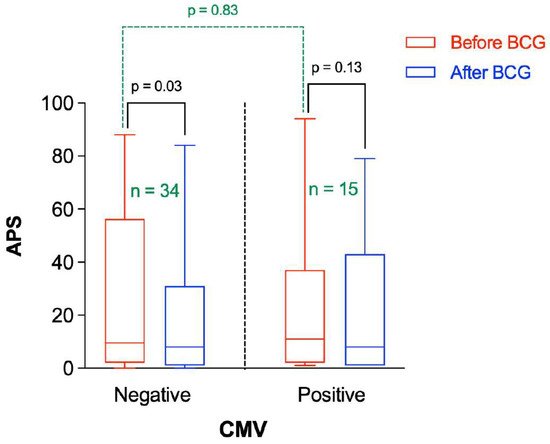

Figure 5. Amyloid Probability Score (APS) before and after BCG vaccination stratified by CMV status. “n” represents the number of observations in each category. Statistical analysis was completed using a Wilcoxon–Pratt Signed-Rank Test for dependent comparisons (solid black) or Mann–Whitney Test for independent comparisons (dashed green).
7. Participants with a Higher CD4/CD8 Ratio Had a Greater Decrease in APS following BCG Vaccination Compared to Those with Lower Baseline CD4/CD8 Ratio
There is a marked decrease in naïve CD4 subsets in patients with AD consistent with the immune system challenged by immunosenescence and/or persistent antigenic challenge [16][15]. The APS before and after BCG vaccination were stratified by the relative CD4/CD8 ratio (Figure 6). As can be seen, there was no significant difference in APS values before BCG vaccine administration between participants having low or high CD4/CD8 ratio (p = 0.52). On average, the APS was significantly lower after BCG administration in participants having a higher CD4/CD8 ratio (p = 0.003), whereas it was not significantly different in participants with a lower CD4/CD8 ratio (p = 0.75) (Figure 6).
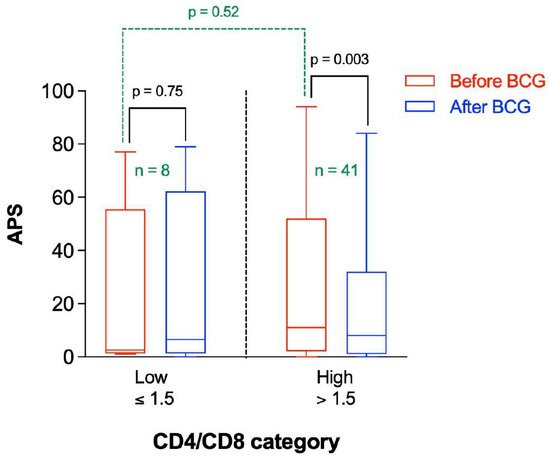

Figure 6. Amyloid Probability Score (APS) before and after BCG vaccination stratified by CD4/CD8 category. n represents the number of observations in each category. Statistical analysis was completed using a Wilcoxon–Pratt Signed-Rank Test for dependent comparisons (solid black) or Mann–Whitney Test for independent comparisons (dashed green).
8. Participants without Latent CMV Infection Had a More “Favorable” “Immune Risk Profile” Than Those with Latent CMV
In elderly individuals positive for CMV there is paucity of naïve T-cells and an accumulation of terminal T-cells with no proliferative capacity [17][16]. This “immune risk profile” manifests in an inverted CD4:CD8 ratio [17,18][16][17]. Thus, the mean CD4/CD8 ratio was measured as a function of the CMV IgG status. As can be seen in Figure 7, the mean CD4/CD8 ratio before vaccine administration was significantly higher in participants having negative CMV IgG compared to participants having positive CMV IgG (mean ± SD = 3.30 ± 1.51 vs. 1.87 ± 0.19, p < 0.0001). The mean CD4/CD8 ratio was not significantly altered in participants following BCG vaccine administration compared to the baseline value (2.86 ± 1.47 vs. 2.84 ± 1.47, p = 0.716).
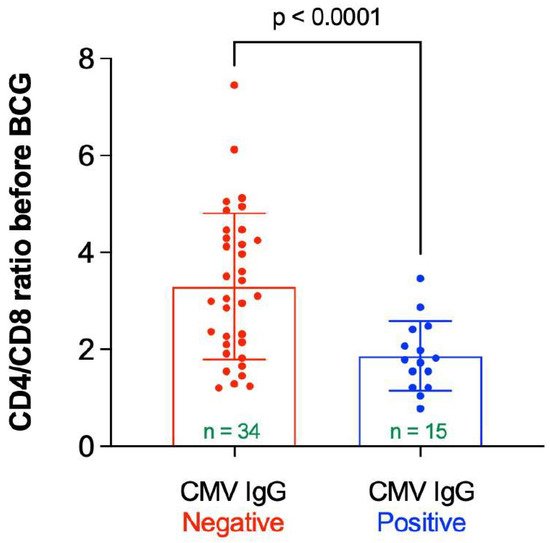

Figure 7. CD4/CD8 ratio before BCG vaccination stratified by status of CMV IgG. “n” represents the number of observations in each category. Statistical analysis was completed using Welch’s t-test.
References
- Calmette, A. Preventive Vaccination Against Tuberculosis with BCG. Proc. R. Soc. Med. 1931, 24, 1481–1490.
- Available online: https://www.ciil.fr/bcg-symposium/scientific-program (accessed on 10 February 2022).
- Gofrit, O.N.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L.; Bercovier, H. Bacillus Calmette-Guérin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS ONE 2019, 14, e0224433.
- Gofrit, O.N.; Bercovier, H.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L. Can immunization with Bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med. Hypotheses 2019, 123, 95–97.
- Zuo, Z.; Qi, F.; Yang, J.; Wang, X.; Wu, Y.; Wen, Y.; Yuan, Q.; Zou, J.; Guo, K.; Bin Yao, Z. Immunization with Bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol. Dis. 2017, 101, 27–39.
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938.
- Jack, C.R.; Therneau, T.M.; Weigand, S.D.; Wiste, H.J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Machulda, M.M.; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging–Alzheimer’s Association Research Framework. JAMA Neurol. 2019, 76, 1174–1183.
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659.
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 34.
- Derhovanessian, E.; Maier, A.; Hähnel, K.; Beck, R.; De Craen, A.J.M.; Slagboom, P.; Westendorp, R.G.J.; Pawelec, G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J. Gen. Virol. 2011, 92, 2746–2756.
- Pawelec, G.; Derhovanessian, E. Role of CMV in immune senescence. Virus Res. 2011, 157, 175–179.
- Lurain, N.S.; Hanson, B.A.; Martinson, J.; Leurgans, S.E.; Landay, A.L.; Bennett, D.A.; Schneider, J.A. Virological and Immunological Characteristics of Human Cytomegalovirus Infection Associated With Alzheimer Disease. J. Infect. Dis. 2013, 208, 564–572.
- Westman, G.; Berglund, D.; Widén, J.; Ingelsson, M.; Korsgren, O.; Lannfelt, L.; Sehlin, D.; Lidehall, A.-K.; Eriksson, B.-M. Increased Inflammatory Response in Cytomegalovirus Seropositive Patients with Alzheimer’s Disease. PLoS ONE 2014, 9, e96779.
- Lövheim, H.; Olsson, J.; Weidung, B.; Johansson, A.; Eriksson, S.; Hallmans, G.; Elgh, F. Interaction between Cytomegalovirus and Herpes Simplex Virus Type 1 Associated with the Risk of Alzheimer’s Disease Development. J. Alzheimers Dis. 2018, 61, 939–945.
- Larbi, A.; Pawelec, G.; Witkowski, J.M.; Schipper, H.M.; Derhovanessian, E.; Goldeck, D.; Fulop, T. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 91–103.
- Pawelec, G.; Koch, S.; Griesemann, H.; Rehbein, A.; Hähnel, K.; Gouttefangeas, C. Immunosenescence, suppression and tumour progression. Cancer Immunol. Immunother. 2006, 55, 981–986.
- Ouyang, Q.; Wagner, W.M.; Wikby, A.; Walter, S.; Aubert, G.; Dodi, A.I.; Travers, P.; Pawelec, G. Large Numbers of Dysfunctional CD8+ T Lymphocytes Bearing Receptors for a Single Dominant CMV Epitope in the Very Old. J. Clin. Immunol. 2003, 23, 247–257.
More
