Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chiara Sanmartin and Version 3 by Vivi Li.
Halophytes represent an ancient and remarkable ecological group of annual or perennial plants with very complex features, whose definition and classification are still not univocal and often controversial. The species belonging to this group are very different from an ecological, morpho–physiological, and taxonomic point of view. Therefore, their definition can be subjective and vary a lot in the literature according to the different interpretations. However, following the most popular definition used in the literature, halophytes can be identified as plants that grow naturally, and complete their life cycle in environments that contain a higher salt content than most plant species can tolerate.
- salt tolerance
- Salicornia
- Suaeda
- Atriplex
- Portulaca
1. Introduction
The occurrence of halophytism is widespread throughout the plant kingdom and appears to have evolved several times, lending support to the concept that many glycophytes may have halophyte genes permanently switched off, but under mutagenesis, may revert to halophytism [1]. In fact, all four plant kingdoms contain members that are adapted to saline habitats; however, they occur in greater numbers among the thallophytes, such as red and brown algae, and terrestrial or aquatic spermatophytes. These plants are mainly distributed in arid or semi-arid inlands and saline wetlands along the tropical and sub-tropical coasts, as well as in temperate zones.
Halophyte plants and their natural habitats have attracted the attention of many naturalists and writers since very remote times. Allusions to salt-tolerant plants in saline soils along the banks and margins of salty waters date back to the mid-1500s, and data on halophytes were already very consistent in the 1700s. In Hortus Cliffortianus and Species Plantarum, when describing plants, Limneo often provides information on the salinity of their habitat [2].
The introduction of the word ‘halophyte’ probably dates to the Russian botanist Peter Simon Pallas; in the early 1800s, he wrote that species of Salicornia, Salsola, Suaeda and related plants, all previously included in the Chenopodiaceae and now included in Amaranthaceae family following APG classification, “must be united under what he believes is an appropriate name for Halophyta” [3]. In contrast to the term ‘halophyte’, the name ‘glycophyte’ was proposed by Stocker (1928) [4] for plants growing in soils with a low salt content. Most cultivated plants are glycophytes. Given the great biodiversity of these plants and the difficulty in distinguishing between salt-tolerant glycophytes and halophytic species, estimating the number of halophyte species and their diffusion is more difficult.
According to Houérou (1993) [5] and Glenn et al. (1999) [6], there are about 6000 species of terrestrial and marine plants in the world that are classified as halophytes. These represent 2% of the angiosperms, and some of them are under continuous taxonomic revision. Of these, approximately 1100 are widespread in the Mediterranean basin, with a wide range of levels of tolerance to salinity. Actually, it is possible to refer to the online database of salt-tolerant plants, namely eHALOPH [7], which is constantly updated. It is based on the records of Aronson (1989) [8] and includes plants that tolerate a soil electrical conductivity (EC) of at least about 8 dS/m, corresponding to about 80 mM NaCl concentrations [9]. The eHALOPH Halophyte Database currently identifies more than 1500 species as salt-tolerant, albeit without labelling them as ‘halophyte’. Other authors report a greater number of halophytic species but, despite the difference, most detailed lists represent only 0.5% of all angiosperms [10].
The complexity of the halophyte group is also linked to the multitude of ecosystems that species can contribute to from all over the world. These habitats can be either natural or non-natural, and include semi-deserts, salt meadows, brackish swamps, mangrove forests, irrigated lands, and even urban areas. Many of the natural habitats are highly threatened due to both natural and anthropogenic factors, such as urbanization, tourism, and pollution. The effects of climate change are increasingly adding to the threats to which natural halophytic habitats and vegetation are exposed. Many saline habitats are likely to disappear due to rising sea levels, as these communities generally do not have the ability to relocate to more inland sites. The conservation value of these ecosystems has recently been assessed in the EU project ‘Red List of habitats’, which are classified as ‘Near Threatened’ (Arctic coastal salt marsh, and Mediterranean and Black Sea coastal salt marsh), ‘Endangered’ (Baltic coastal meadow) or ‘Vulnerable’ (Atlantic coastal salt marsh) [11]. In addition, many countries are facing the loss of agricultural soil due to secondary salinization resulting from irrigation, fertilization, and climate change. Nearly 20% of the total irrigated land area (45 million hectares) is already affected by salinization [12]. In regions with saline soils, halophytes could be alternative crops to glycophytes to produce food, forage, and fibers, and for industrial purposes. Moreover, when halophytes are grown as companion plants of glycophytes, the negative effect of salinity is alleviated by the uptake of toxic ions by the former species, as found by Colla et al. (2006) [13] and Karakas et al. 2021 [14].
2. Classification of Halophytes
Halophytes are very peculiar both physiologically and morphologically, and in some cases, are of great phytogeographic interest. It is, therefore, not surprising that they have been the subject of many studies in various fields of botany, and still are today.
The survival of halophytes is strongly influenced by the salinity in the root zone or, sometimes also by salt spray, as this can happen in saline semi-deserts, in brackish marshes, or on beaches. These types of environments have both negative effects on plants, due to the high osmotic pressure of the soil solution and the toxic effect of salt, and positive ones, as it reduces competition with other plants and may prevent diseases and parasites [15].
When a plant’s response to salinity is considered, rwesearchers usually refer to NaCl, although halophytes are also strongly influenced by other salts, such as MgSO4, NaSO4 or KCl. Halophytes can be also described as plants that survive in environments where the salt concentration is around 200 mM NaCl or more [16]. For example, the most salt-tolerant halophytes, such as Suaeda salsa, can complete their life cycle in soils containing 200 to 500 mM NaCl [17]. Many studies have also shown that suitable high salt concentrations can enhance the growth of halophytes in comparison with low or negligible salinity [18][19]. This is proven at both the morphological and physiological level, with a greater biomass accumulation and better seed quality [20], and a higher photosynthetic efficiency [21][22].
By contrast, glycophytes have a limited tolerance to salinity, showing significant reductions in biomass under saline conditions. Among the glycophytes, two types are described based on their degree of sensitivity: salt-sensitive and salt-tolerant. Glycopyhytes include species, such as rice or soybean, that can suffer irreparable damage starting from very low concentrations (less than NaCl 50 mM), and others, such as barley or beets, that can tolerate much higher NaCl concentrations (even those close to 200 mM) [23]. From the morpho–functional point of view, no single factor is of major importance for the ability to survive at high NaCl salinity. Therefore, there are no peculiar characteristics that univocally define all the halophytic species, being a very heterogeneous and systematically complex group. The combination of several mechanisms often enhances the salt tolerance of individual species [24].
However, the evolution of multiple specific morpho-anatomical adaptations and functional characteristics is evident, aimed at fighting the saline environment and even benefiting from it. These adaptations are mostly related to the prevention of leaf water loss by transpiration, with dilution of the salts absorbed in excess or, in some cases, with their excretion. These characteristics are found mainly in the leaves and stems, since they are the plant organs most rigorously exposed to environmental conditions. Among the most important characteristics may be the reduction or absence of leaves, the abundance of glandular trichomes or saline glands, the greater development of supporting tissues, the development of atypical secondary growth, and leaf succulence. Typical succulent halophytes are, for example, species of Salicornia and Sarcocornia; these are two very interesting genera that appeared in Eurasia in the Middle Miocene period, and that have long represented difficulties for taxonomists due to their growth form with very small leaves and flowers. Although the response of halophytes to the multiple stressors of saline environments is well known (Figure 1), their classification in relation to the total number of flowering plants [25] continues to be very uncertain.
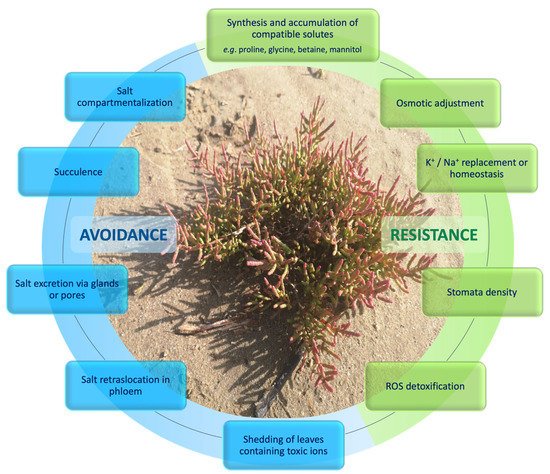

Figure 1.
Response mechanisms to salinity stress by halophytic plants. Photo credit: Tiziana Lombardi.
As previously anticipated, due to their taxonomic and ecological complexity, even today, there is no consensus on an univocal definition. It is, therefore, difficult to draw a complete list of halophyte species, partly due to the problem of defining the lower limit of salt tolerance at which a plant should be considered a halophyte. Of course, this difficulty is reflected at the environmental level, in the vegetational subdivisions and zonation of communities dominated by halophyte species.
Halophytes are classified in various ways based on multiple factors, such as general ecological behaviour and distribution; plant growth response to salinity; salt intake rate; the presence or absence of saline glands, physiotype, etc. [15].
2.1. Response to Internal Salt Content
One of the first proposed classifications for flowering plants was based on their response to internal salt content, and divides halophytes into salt-regulating or and salt-accumulating types [26]. Based on their internal salt contents, halophytes were later also classified as excluders versus includers.
2.2. Eco-Physiological Aspects
Eco-physiological aspects can be used to differentiate the halophytes in obligate, facultative, and habitat-indifferent species . This classification is based on what Ingram introduced in 1957, albeit without any definition of “low”, “moderate”, and “high” salinity. All three types of halophytes have better growth and development than glycophytes in saline environments.
-
Obligate halophytes, mostly the Amaranthaceae members, are species with optimal growth at moderate or high salinity (NaCl 0.1–5.0%); they cannot grow at lower salinity, as they require salt as part of their nutrition to activate or de-activate several salt-sensitive enzymes. These species frequently exhibit an activation of these enzymes, both at very low Na+ concentrations (below the physiological optimum) and at seawater Na
-
Eu+ concentrations (considered excessive).
- -halophytes (extreme halophytes) are plants that can grow in seawater or tolerate more than 200 mM NaCl (up 5%), and occur almost exclusively in environments of high salinity [9]. Following the eHALOPH database, this group contains 333 species, members of 70 families of flowering plants. The 75% of eu-halophytes belong to just 19 families. Eu-halophytes are rather rare amongst flowering plants, representing just 0.4% of the 350,699 accepted names in ‘The Plant List’ within 20% of its 642 families. Some species of Atriplex, Salicornia, Suaeda, and
Based on the different mechanism of salt tolerance, halophytes can be further classified as follows [29]:
-
Salt-excludingSalsola
-
Facultative halophytes are plants with the ability to grow on salty soils, but their optimal growth is observed in low-salt or non-saline conditions. Many dicotyledons such as Aster tripolium, Chenopodium quinoa, Glaux maritima, and Plantago maritima, but also some Poaceae, Cyperaceae and Juncaceae, belong to this group.
-
Habitat-indifferent halophytes normally grow on salt-free substrates, but in saline conditions they thrive better than sensitive species. This group includes the plants that can live in disturbed or stable habitats. In some of these, such as Festuca rubra, Agrostis stolonifera and Juncus bufonius, there are significant genetic and morphological differences between the populations living on salty soils and those on salt-free soils.
2.3. Salt Tolerance
According to their salt tolerance, Chapman (1942) [27]
- can be included in this group.
-
Mio-halophytes are plants that grow in habitats with low levels of salinity (less than 0.5% NaCl).
Much later, Grigore and Toma (2010) [28] proposed a new classification of halophytes, distinguishing the extreme halophytes from meso-halophytes, and dividing the extreme halophytes between irreversible and reversible. Thus, based on the analysis of the anatomical features of some taxa and ecological factors, they concluded that succulent Amaranthaceae (e.g., Salicornia europaea, Suaeda maritima
- (root-excluding type) are halophytes (also known as pseudo-halophytes) that protect the shoot from salinity through apoplastic barriers in the roots and interveinal recycling of ions. Mangrove vegetation shows such a type of tolerance.
-
Salt-excreting (endo- and eso-recretohalophytes) are plants that avoid cellular damage by releasing excess salts to the outside via specialized structures called salt glands—such as species of Limonium, Tamarix, Spartina, Avicennia, and Frankenia—or from epidermal bladders on the leaves, such as species of Atriplex and Chenopodium.
-
Salt-accumulating are plants able to accumulate salts that are compartmentalized into vacuoles and used for osmotic adjustment, e.g., Salvadora persica, Sesuvium portulacastrum, Suaeda nudiflora.
2.4. Habitat and Geographical Distribution
The classification based on the habitat and geographical distribution includes hydro-halophytes and xero-halophytes [30]:
-
Hydro-halophytes are halophytic plants that need aquatic conditions or wet soil. Species growing in aquatic environments belong to this group, such as the mangrove forests, tidal marshes or coastal lagoons, and the brackish marshes of the temperate zone. Zannichellia palustris and Althenia filiformis are typical hydro-halophytes in the Mediterranean area [31][32].
-
Xero-halophytes grow in environments with dry soil due to high evapotranspiration. Most plants living in desert areas and succulents belong to this group. Atriplex canescens or A. halimus are xero-halophytes that tolerate both salt and drought stress.
3. Mediterranean Halophytes
In the Mediterranean regions, Amaranthaceae are the dominant angiosperm family, with halophyte species such as Salicornia sp., Sarcocornia sp., Suaeda sp., Salsola sp. and Atriplex sp. They are followed by Poaceae, such as Hordeum sp., Puccinellia sp., and Sporobolus sp. [8][9] (Table 1). Halophyte species are mostly found in coastal brackish areas that are subjected to periodic flooding.
Table 1. List of halophytes selected on the basis of their diffusion in the Mediterranean region and their type of halophytism according to eHALOPH [7].
| Botanical Name | Family | Common Name in English, French, German and Italian | Type of Halophytism | Edible Organs |
|---|---|---|---|---|
| Atriplex littoralis L. (Syn.: Atriplex patula L. var. littoralis(L.) A. Gray) | Amaranthaceae | Grassleaf orache, Arroche du littoral, Strand-meide, Atriplice litorale. | Psammophyte | Leaves |
| Atriplex prostrata Boucher ex DC (Syn.:Atriplex latifolia Wahlenb. = Atriplex hastata L. var. prostrata (Boucher ex DC.) Lange) | Amaranthaceae | Hastate orache, Arroche couché, Spiess-meide, Atriplice prostata | Eu-halophyte Meso-hydrohalophile |
Leaves |
| Beta vulgaris L. subsp. maritima (L.) Arcang. (Tuscany, Sardinia, Sicily) | Amaranthaceae | Sea beet, Bette maritime, Wilde übe, Bietola marittima | Mio-halophyte | Leaves |
| Cakile maritima Scop. subsp. maritima | Brassicaceae | Searocket, Roquette de mer, Strandrauke, Ravastrello di mare | Psammophile Halo-nitrophilous |
Leaves |
| Halimione portulacoides (L.) Aellen (Syn.: Atriplex portulacoides L.) | Amaranthaceae | Sea pursiane, Arroche faux-pourpier, Strand-salzmeide, Porcellana di mare | Eu-halophyte Hydro-halophyte |
Leaves |
| Portulaca oleracea L. subsp. oleracea | Portulacaceae | Common pursiane, Purcelane, Portulach, Porcellana | Xero-halophyte | Leaves Stem |
| Salicornia perennans Willd. subsp. perennans (Syn.: Salicornia europaea auct.; Salicornia patula Duval-Jouve). | Amaranthaceae | Grasswort, Salicorne etaleé, Pannonien glasschmaiz, Salicornia patula | Eu-halophyte Xero-halophyte |
Stem |
| Salicornia perennis Mill. subsp. perennis (Syn.: Sarcocornia perennis (Mill.) A.J.Scott subsp. perennis). | Amaranthaceae | Perennial grasswort, Salicorne vivace, Ausdauernde gliedermeide, Salicornia radicante | Eu-halophyte Hydro-halophyte |
Stem |
| Salsola soda L. (Syn: Soda inermis Fourr.) | Amaranthaceae | Monk’s beard, Soude commune, Soda-salzicraut, Agretto | Eu-halophyte | Leaves Young stem |
| Suaeda maritima (L.) Dumort. (Syn.: Chenopodium maritimum L.) | Amaranthaceae | Sea-blite, Soude maritime, Strand-sode, Sueda marittima | Eu-halophyte Mesohydro-halophile |
Leaves Young stem |
| Suaeda vera J.F. Gmel (Syn.: Suaeda fruticosa (L.) Forssk. subsp. vera (J.F. Gmel.) Maire & Weiller). | Amaranthaceae | Shrubby sea-blite, Soude vraie, Strauchige sode, Sueda vera | Eu-halophyte Halo-nitrophilous |
Leaves Young stem |
The choice took advantage of both their diffusion and importance at an environmental level in the Mediterranean regions, and their degree of salt tolerance recognized by the main international databases such as eHALOPH. The species were then characterized from a botanical point of view and investigated regarding the availability of data on their potential as food-crop sources. Some data have also been provided on the distribution and availability of these species on the Italian territory, with reference to the Tyrrhenian coasts [33][34] (Figure 2 a, b). These areas represent an important germplasm reserve for the use of local varieties or ecotypes. The species are reported with the scientific and common name, the type of halophytism, and the edible organs.


Figure 2. Salt marsh vegetation of Tyrrhenian coasts (Italy): (a) Salicornia perennis communities; (b) Salicornia perennans communities. Photo credit: Andrea Bertacchi.
For all the species considered, reswearchers followed the taxonomic nomenclature reported by Bartolucci et al. (2018) [35], except for Soda inermis, herein kept as Salsola soda. This is due to the taxonomic difficulties of not being fully clarified by the Angiosperm Phylogeny Group classification [36], related to the revisions of the genus Salsola, from which the taxon Soda was separated with the only species Soda inermis.
The species and subspecies Halimione portulacoides, Salicornia perennans subsp. perennans, Salicornia perennis subsp. perennis, Salsola soda, Suaeda maritima and S. vera are exclusive to brackish areas. A. prostrata, B. vulgaris subsp. maritima, and Portulaca oleracea can be found in both brackish areas and internal saline soils. Cakile maritima subsp. maritima, and Atriplex littoralis are halophytes that grow exclusively in dune environments.
This entry was conducted in the framework of the project entitled “HALOphytes grown in saline Water for the production of INnovative ready-to- eat salad-HALOWIN” and funded by the University of Pisa, “PRA 2020”, grant number PRA_2020_43.
References
- Yensen, N.P. Halophyte Uses for the Twenty-First Century. In Ecophysiology of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 2006; pp. 367–396.
- Uphof, J.C.T. Halophytes. Bot. Rev. 1941, 7, 1–58.
- Strogonov, B.M. Structure and Function of Plant Cells in Saline Habitats-New Trends in the Study of Salt Tolerance (Translated from Russian by A. Mercado); John Wiley and Sons: New York, NY, USA, 1973.
- Stocker, O. Das Halophyten Problem. In Ergebnisse der Biologie; Springer: Berlin/Heidelberg, Germany, 1928; pp. 265–353.
- Lielh, H.; Al, A.; le Houerou, R.N. Salt-Tolerant Plants for the Arid Regions of the Mediterranean Isoclimatic Zone; Springer: Dordrecht, The Netherlands, 1993.
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255.
- The University of Sussex and Other Contributors EHALOPH—Halophytes Database—Version 3.22. Available online: https://www.sussex.ac.uk/affiliates/halophytes/index.php (accessed on 3 November 2021).
- Aronson, J.A. HALOPH: A Data Base of Salt Tolerant Plants of the World, 1st ed.; Office of Arid Lands Studies, University of Arizona: Tucson, AZ, USA, 1989.
- Santos, J.; Al-Azzawi, M.; Aronson, J.; Flowers, T.J. EHALOPH a Database of Salt-Tolerant Plants: Helping Put Halophytes to Work. Plant Cell Physiol. 2016, 57, e10.
- Cheeseman, J.M. The Evolution of Halophytes, Glycophytes and Crops, and Its Implications for Food Security under Saline Conditions. New Phytol. 2015, 206, 557–570.
- Janssen, J.A.M.; Rodwell, J.S.; García Criado, M.; Gubbay, S.; Haynes, T.; Nieto, A.; Sanders, N.; Calix, M. European Red List of Habitats, 1st ed.; Commission, E., Ed.; Publications Office of the European Union: Luxembourg, 2016; ISBN 9789279615887.
- FAO. Salt-Affected Soils. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/2018 (accessed on 15 September 2021).
- Colla, G.; Roupahel, Y.; Cardarelli, M.; Rea, E. Effect of Salinity on Yield, Fruit Quality, Leaf Gas Exchange, and Mineral Composition of Grafted Watermelon Plants. HortScience 2006, 41, 622–627.
- Karakas, S.; Bolat, I.; Dikilitas, M. The Use of Halophytic Companion Plant (Portulaca oleracea L.) on Some Growth, Fruit, and Biochemical Parameters of Strawberry Plants under Salt Stress. Horticulturae 2021, 7, 63.
- Waisel, Y. Biology of Halophytes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 410.
- Flowers, T.J.; Colmer, T.D. Plant Salt Tolerance: Adaptations in Halophytes. Ann. Bot. 2015, 115, 327–331.
- Guo, J.; Suo, S.; Wang, B.-S. Sodium Chloride Improves Seed Vigour of the Euhalophyte Suaeda salsa. Seed Sci. Res. 2015, 25, 335–344.
- Debez, A.; Hamed, K.B.; Grignon, C.; Abdelly, C. Salinity Effects on Germination, Growth, and Seed Production of the Halophyte Cakile maritima. Plant Soil 2004, 262, 179–189.
- Zhang, H.; Irving, L.J.; Tian, Y.; Zhou, D. Influence of Salinity and Temperature on Seed Germination Rate and the Hydrotime Model Parameters for the Halophyte, Chloris virgata, and the Glycophyte, Digitaria sanguinalis. S. Afr. J. Bot. 2012, 78, 203–210.
- Guo, J.; Du, M.; Lu, C.; Wang, B. NaCl Improves Reproduction by Enhancing Starch Accumulation in the Ovules of the Euhalophyte Suaeda salsa. BMC Plant Biol. 2020, 20, 262.
- Zhang, S.; Song, J.; Wang, H.; Feng, G. Effect of Salinity on Seed Germination, Ion Content and Photosynthesis of Cotyledons in Halophytes or Xerophyte Growing in Central Asia. J. Plant Ecol. 2010, 3, 259–267.
- Balnokin, Y.V.; Kurkova, E.B.; Myasoedov, N.A.; Lun’kov, R.V.; Shamsutdinov, N.Z.; Egorova, E.A.; Bukhov, N.G. Structural and Functional State of Thylakoids in a Halophyte Suaeda altissima before and after Disturbance of Salt-Water Balance by Extremely High Concentrations of NaCl. Russ. J. Plant Physiol. 2004, 51, 815–821.
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive Physiology of Halophytes: Current Standing. Front. Plant Sci. 2019, 9, 1954.
- Koyro, H.-W.; Geißler, N.; Hussin, S.; Huchzermeyer, B. Survival at Extreme Locations: Life Strategies of Halophytes—The Long Way from System Ecology, Whole Plant Physiology, Cell Biochemistry and Molecular Aspects Back to Sustainable Utilization at Field Sites. In Biosaline Agriculture and High Salinity Tolerance; Birkhäuser: Basel, Switzerland, 2008; pp. 1–20.
- Flowers, T.J.; Muscolo, A. Introduction to the Special Issue: Halophytes in a Changing World. AoB Plants 2015, 7, plv020.
- Steiner, M. To the Ecology of the Salt March of the Nordost lichen United Countries of Nordamerika. Jahrb. Know Offer. 1934, 81, 94.
- Chapman, V.J. The New Perspective in the Halophytes. Q. Rev. Biol. 1942, 17, 291–311.
- Grigore, M.; Toma, C. A Proposal for a New Halophytes Classification, Based on Integrative Anatomy Observations. Muz Olten. Craiova Stud. Şi Comun. Ştiinţele Nat. 2010, 26, 45–50.
- Walter, H. Salinity Problems in the Acid Zones. The Adaptations of Plants to Saline Soils. Arid Zone Res. 1961, 14, 65–68.
- Aslam, R.; Bostan, N.; Nabgha-e-Amen; Maria, M.; Safdar, W. A Critical Review on Halophytes: Salt Tolerant Plants. J. Med. Plant Res. 2011, 5, 7108–7118.
- Lombardi, T.; Bedini, S.; Bertacchi, A. Germination Ecology of the Aromatic Halophyte Artemisia caerulescens L.: Influence of Abiotic Factors and Seed after-Ripening Time. Folia Geobot. 2019, 54, 115–124.
- Lombardi, T.; Bedini, S. Seed Germination Strategies of Mediterranean Halophytes under Saline Condition. In Handbook of Halophytes; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19.
- Bertacchi, A.; Lombardi, T.; Saggese, A.; Lazzeri, V. The Vegetation of a Relict Salt Marsh Area in the Pisan Coast in the Context of Brackish Wetlands of Tuscany. Plant Sociol. 2021, 58, 41–53.
- Frondoni, R.; Iberite, M. The Halophile Vegetation of the Sedimentary Coast of Lazio (Central Tyrrhenian District, Italy). Plant Biosyst. 2002, 136, 49–67.
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An Updated Checklist of the Vascular Flora Native to Italy. Plant Biosyst. 2018, 152, 179–303.
- Byng, J.W.; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Judd, W.S.; Mabberley, D.J.; Sennikov, A.N.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F.; et al. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. The Angiosperm Phylogeny Group. Bot. J. Linn. Soc. 2016, 35, 1–20.
More
