Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kultigin Turkmen and Version 2 by Amina Yu.
Cardiovascular diseases remain the most common cause of morbidity and mortality in chronic kidney disease patients undergoing hemodialysis. Epicardial adipose tissue (EAT), visceral fat depot of the heart, was found to be associated with coronary artery disease in cardiac and non-cardiac patients. Additionally, EAT has been proposed as a novel cardiovascular risk in the general population and in end-stage renal disease patients. It has also been shown that EAT, more than other subcutaneous adipose tissue deposits, acts as a highly active organ producing several bioactive adipokines, and proinflammatory and proatherogenic cytokines.
- epicardial adipose tissue
- cardiovascular morbidity and mortality
- hemodialysis
1. Relationship between CVD and EAT in End Stage Renal Disease (ESRD) RD Patients and Patients Receiving Hemodialysis Treatment
Diabetes and hypertension are the most common causes of chronic renal failure, and the presence of these diseases, in addition to kidney failure, poses an additional cardiovascular risk. Cardiovascular diseases account for nearly half of all deaths in CKD patients, and a history of CKD increases the risk of cardiovascular disease by 1.5 to 3.5 times [1][2][67,68]. Dialysis patients have a higher rate of coronary artery disease, congestive heart failure, sudden death, and arrhythmias than the general population [3][69].
Traditional risk factors such as advanced age, smoking, obesity, dyslipidemia, and family history are insufficient to explain why CKD patients have an increased risk of CVD. Common metabolic disorders in CKD patients include oxidative stress, nonspecific chronic inflammation, uremic toxins, anemia, malnutrition, insulin resistance, metabolic acidosis, hyperparathyroidism, hyperhomocystinemia, and vitamin D deficiency [2][4][68,70]. Atherosclerosis, endothelial dysfunction, hypertension, diabetes mellitus (DM), and micro-macrovascular complications caused by diabetes, chronic inflammation, coronary artery calcification (CAC), and left ventricular hypertrophy (LVH) are the most important risk factors for CVD in patients with chronic kidney disease [5][6][71,72]. Atherosclerosis, inflammation, and vascular calcification are the most common risk factors in the pathogenesis of CVD in ESRD patients [5][71] (Figure 12).
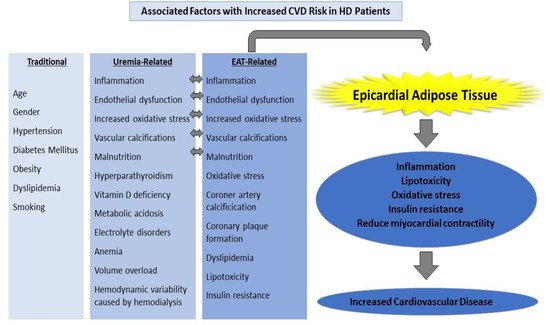
Figure 12.
Factors associated with an increased CVD risk in HD patients.
End-stage renal disease is distinguished by a persistent and non-specific inflammatory process. Many inflammatory cytokines, especially TNF-α, IL-1-β, and IL-6 increase in CKD [7][8][73,74]. Inflammatory cytokines are linked to endothelial dysfunction, atherosclerosis, and, eventually, an increase in cardiovascular disease. Hemodialysis, a renal replacement therapy used in end-stage renal disease, is associated with additional risks such as increased oxidative stress and endothelial dysfunction, as well as cardiovascular risks caused by kidney failure. Hemodynamic instability during dialysis is another significant event that raises cardiovascular risk [9][75].
Dialysis patients have a higher rate of coronary artery disease, congestive heart failure, sudden death, and arrhythmias than the general population [3][69]. Traditional risk factors such as age, gender, hypertension, obesity, dyslipidemia, or smoking alone cannot explain the increased frequency of CVD in dialysis patients. Inflammation, endothelial dysfunction, increased oxidative stress, and vascular calcifications are cited as major causes of these elevated CVD risks. Malnutrition, hyperparathyroidism, vitamin D deficiency, long-term and severe uremia, metabolic acidosis, electrolyte disorders, anemia, long-term volume overload, and hemodynamic variability caused by hemodialysis all contribute to an increase in the incidence of cardiovascular disease [2][4][5][6][68,70,71,72]. Chronic kidney disease itself is also considered a CVD risk equivalent.
DIn a study of diabetic kidney disease patients conducted by Sasso et al. [10][76], found thate incidence of fatal/non-fatal CVD in patients who received multifactorial intensive therapy against cardiovascular disease risk factors was 53% lower than in the group that received standard treatment. Int this study, intensified and standard treatments were compared against known standard and manageable risk factors for CVD. It This study compared intensified and standard treatments to known standard and manageable risk factors for CVD. [11][77]. However, it iswe unknownknow that dialysis patients consume more EAT than the general population. EAT was linked to many known risk factors in this patient group, including age, low HDL cholesterol, high LDL cholesterol and triglyceride levels, smoking, and BMI [12][13][14][15][30,44,52,53]. The increased EAT volume in dialysis patients can be viewed as both a result and a cause of these pathways, which increase the frequency of CVD. In HD patients, the relationship between EAT and CVD is primarily focused on epicardial adipose tissue enlargement, inflammation, and hypermetabolic activity, which is led by systemic inflammation [16][78].
In a recent study, 221 ESRD patients were tested to see if EAT radiodensity and volume could predict long-term mortality. In patients with ESRD, EAT radiodensity was an independent predictor of all-cause mortality. EAT volume, on the other hand, was not linked to mortality [17][79]. IThis st udy is useful in terms of linking EAT to long-term mortality. Similarly, in another study examining the relationship of EAT thickness with mortality in ESRD patients over a 10-year period, patients with an EAT thickness greater than 11.45 mm lived approximately 2 years longer than those with an EAT thickness less than 11.45 mm [18][80].
In the general population, there is a positive relationship between age and the amount of EAT consumed [19][81]. When a similar relationship was investigated in HD patients, it was discovered that the amount of EAT was also related to increasing age [20][82]. There is a redistribution of visceral fat stores with aging, which is thought to increase the amount of EAT.
A 4-year follow-up period was included in the first study to describe the relationship between EAT and mortality in hemodialysis patients. EAT volume has been shown to be an independent predictor of mortality in hemodialysis patients. The study found that every 10cc increase in EAT volume resulted in a 6% increase in the risk of death. Researchers believe that this increased risk is due to EAT’s effects on vasculopathy and CAC [12][30]. CAC is a type of long-term vascular calcification that can be detected early in ESRD patients and is thought to contribute to both increased CVD and mortality. We previously demonstrated a link between EAT and coronary artery calcification in patients with end-stage renal disease receiving hemodialysis and peritoneal dialysis in three studies [21][22][23][39,61,64]. EAT was found to be associated with vascular calcification in HD patients in another study with a similar design, but it could not be determined as a predictor of mortality [24][83]. The smaller number of patients in this study reduces the study’s data reliability.
2. Risk Factors
The most common risk factors in the pathogenesis of CVD in dialysis patients are atherosclerosis, inflammation, and vascular calcification [5][71]. Coronary flow reserve is regarded as a marker of endothelial dysfunction. EAT thickness in hemodialysis patients was found to be inversely proportional to CFR in a study examining the relationship between EAT and coronary flow reserve [25][84]. Coronary flow reserve is regarded as a marker of endothelial dysfunction. EAT thickness in hemodialysis patients was found to be inversely proportional to CFR in a study examining the relationship between EAT and coronary flow reserve [26][27][28][29][15,17,18,20]. EAT is not usually found symmetrically around the heart. Atherosclerotic plaques are becoming more common, particularly in areas where EAT accumulation is high [29][20]. This raises the possibility that EAT is causing inflammation through a paracrine effect [30][35]. This hypothesis is supported by the observation that EAT thickness is significantly associated with the presence and severity of CAD, as well as by the ability to stop CAD progression following epicardium resection [31][85].
The amount of EAT increases as the stage of renal failure progresses. It is well known that dialysis patients consume more EAT than the general population. Saritas et al. [32][27] discovered that eGFR was independently and inversely related to EAT volume, and they hypothesized that EAT could be a marker of the uremia-specific component of CV risk based on this finding.
In the comparison of hemodialysis patients and transplant patients at the same follow-up period, EAT levels were found to be higher in dialysis patients and associated with inflammatory variables; EAT levels in transplant patients were found to be both unrelated to inflammatory markers and similar to the healthy population. This clearly demonstrates the role of increased inflammatory processes in dialysis patients in the EAT and CVD equation. [33][56]
Obese people consume more calories. Obesity is a risk factor for CVD but not an independent predictor of EAT [34][6]. Dialysis patients are at risk of malnutrition due to reduced food intake and increased inflammation. Weight loss due to malnutrition and dialysis is linked to an increased risk of death. Muscle wasting, which occurs as catabolic processes increase, leads to an increase in insulin resistance [35][62]. Simultaneously, the combination of increased uremia and decreased GFR alters the distribution of visceral adipose tissue [36][59]. TheOur group’s two studies clearly demonstrated the link between malnutrition and EAT in HD patients. In the first study, a link was our group discovered a link between low albumin levels and increased EAT accumulation in HD patients; in the second study, a link was we discovered a link between EAT and malnutrition, elevated inflammatory markers, atherosclerosis, and vascular calcification in HD patients [22][37][61,86]. Despite having a low BMI, HD patients are at an increased risk of EAT accumulation and CVD [22][61]. Insulin resistance caused by malnutrition in dialysis patients increases metabolic events and CVD risks. While visceral adipocyte tissue accumulation is not an independent risk factor for cardiovascular events, there are numerous studies showing that EAT is an independent predictor of cardiovascular events [38][39][12][40][28,29,30,31]. Furthermore, EAT produces more inflammatory cytokines than subcutaneous adipose tissue [41][21]. Based on these findings, EAT accumulation in dialysis patients is more valuable than other visceral adipose tissue deposits in predicting increased CVD frequency, and it is independent of body weight.
Comorbidity diseases such as diabetes and hypertension that accompany this patient group are one of the most important reasons for the high CVD risks in dialysis patients. Diabetes and hypertension patients consume more EAT than the general population. The amount of EAT is higher in diabetic and hypertensive individuals than in the healthy population [34][6]. Different results have been obtained in studies conducted on whether diabetes affects the amount of EAT in renal failure. Various studies on whether diabetes affects the amount of EAT in renal failure have yielded conflicting results. While Tonbul et al. [42][63] discovered that EAT was higher in diabetic kidney patients than in non-diabetic patients, Mazurek et al. [43][3] demonstrated that the relationship between EAT and CAD in CKD is independent of diabetic status and suggested that there are other factors other than diabetes that influence the amount of EAT in CKD patients. While Tonbul et al. found EAT higher in diabetic kidney patients than in non-diabetic patients, Mazurek et al. showed that the relationship between EAT and CAD in CKD is independent of diabetic status and suggested that there are additional reasons independent of diabetes in determining the amount of EAT in CKD patients. There is substantial evidence in the literature that systolic blood pressure and LVH are related to EAT thickness [44][87]. Hypertension (HT) is common in patients with end-stage renal disease (ESRD). The increase in EAT mass to meet the increased myocardial energy requirement after the increase in left ventricular wall thickness caused by high blood pressure is one of the most important mechanisms that can explain the relationship between EAT and LVH [45][88].
Dyslipidemia is a metabolic disorder that is commonly found in dialysis patients and is a known risk factor for CVD. EAT in dialysis patients was found to be inversely correlated with HDL cholesterol and positively correlated with LDL cholesterol and TG in studies examining the amount of EAT and cholesterol levels [32][12][13][14][15][27,30,44,52,53]. The link between hyperlipidemia and EAT is well established, and the situation in dialysis patients is similar to that of the general population.
3. Potential Solutions
Exercise lowers the risk of CVD as well as the amount of EAT. The only study that looked at the relationship between exercise and the amount of EAT in dialysis patients discovered that standard exercise on days when HD treatment was not given reduced the amount of EAT [14][52]. The authors interpreted the possible cause of this situation as decreased EAT thickness due to decreased oxidative stress.
Between dialysis sessions, dialysis patients with insufficient renal residual function experience an average of 3 kg of hypervolemia. LVH develops over time as a result of the chronic hypervolemia state. EAT and hypervolemia were found to be correlated in the only study that looked at the relationship between hypervolemia and EAT [33][56]. It is possible that increased hypervolemia causes LVH and that this condition creates an inducing force on EAT accumulation due to myocytes’ increased energy requirement.
Not only is there a link between EAT and coronary artery disease, but there is also a link between heart failure and arrhythmia and EAT, according to research. There was a significant relationship discovered between the amount of EAT and atrial fibrillation [46][54]. EAT is thought to cause arrhythmias due to the pressure it exerts on surrounding tissue, and it is also thought to cause heart failure due to the oxidative stress it causes on the myocardium [27][47][17,22].
The accumulation of EAT increases in direct proportion to the duration of HD treatment [48][65]. The findings of studies looking into the effects of reducing uremia by increasing hemodialysis treatment on EAT are contradictory [49][50][58,66]. This difference may be due to the presence of different diseases affecting the amount of EAT in the patient groups, as well as differences in the patients’ dialysis treatment history, and it supports the idea that reducing uremia alone cannot reduce CVD risks without reducing existing inflammation [51][57].
Corrective lifestyle changes and medical treatments for known CVD risk factors reduce EAT as well. Severe dietary restrictions cause a greater reduction in EAT in obese individuals than BMI and waist circumference [52][89]. Treatment modalities that lower lipid and glucose levels also cause a decrease in the amount of EAT [53][90]. Statin therapy was found to have a greater anti-inflammatory effect on EAT than subcutaneous adipose tissue [54][91].
