The consumption of prickly pear (PP; Opuntia spp.) cladodes was reported to exert hypoglycemic effects, making it a potential cost-effective nutritional intervention for the management of T2DM. Several studies have demonstrated that the consumption of prickly pear cladodes and the related products reduced post-prandial glucose levels. The cladodes’ high fiber content may be implicated in improving glycemic control, by affecting glucose absorption and effectively slowing its release into the blood circulation. Given these potential hypoglycemic effects, prickly pear cladodes may represent a potential functional food ingredient to improve glycemic control and counter the negative metabolic effects of the modern Western diet.
- prickly pear
- cladode
- Opuntia spp.
- hypoglycemia
- hyperglycemia
- type 2 diabetes mellitus
- blood glucose
1. Introduction
2. Etiology of Type 2 Diabetes
3. Prickly Pear Cacti: General Information and Composition
The PP cacti are a resistant desert species native to the American regions, and, due to their high adaptability, Opuntia spp. have spread to other regions around the globe, such as Europe [27] and Australia [8][12][28]. The annual production of PP exceeds 400,000 metric tons in Mexico alone [29], where it is traditionally consumed as a vegetable [27]. Furthermore, the cladodes are typically consumed, whether broiled, blended, or as a juice after the removal of the spines [30][31]. The main components in cladodes are carbohydrates (38% dry weight (d.w.)), proteins (11% d.w.) and water (83%; 5:1 biomass to water ratio) [32][33]. The nutritional composition may differ among Opuntia spp., dependent on the specimen’s age and environmental factors, such as the cultivation season and geographical position [33]. The primary polysaccharide in cladode is mucilage, an ingredient commonly used in the food industry as an additive and an emulsifier [33]. Furthermore, an analysis of cladode extract indicated the presence of several phytochemicals, mainly polyphenols and phenolic acids [31], all of which have been implicated in certain beneficial health outcomes particularly related to the management of CVD [34][35][36][37].4. Anti-Hyperglycemic Effect of the Prickly Pear Cladode
The findings from several different randomized controlled trials (Table 1) have indicated a potential (mainly acute) hypoglycemic effect immediately following the consumption of cladodes. A study conducted by Frati et al. [14] aimed to evaluate the potential hypoglycemic effects of different cladode preparations (500 g; broiled, blended and broiled, blended crude, and heated, blended crude) after ingestion, in participants diagnosed with T2DM (n = 8). It was observed that all methods of cladode preparation resulted in an acute reduction of blood glucose at 120 and 180 min following the cladode intake (p < 0.01). The peak hypoglycemic effect of the cladode intervention observed a reduction ranging from 23.3 ± 4.4 mg/dL to 25.4 ± 14.3 mg/dL. No difference was noted in the hypoglycemic effectiveness between the preparation methods of the cladode (p > 0.05). A similar outcome was also observed in an earlier study by Frati-Munari et al. [38].| Reference | Aim/s | Participants | Intervention/Design | Results | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frati et al. | [14] | To assess the effect of | Opuntia ficus indica | cladode on hyperglycemia in T2DM subjects. | T2DM participants ( | n | = 8; 2 M and 6 F; mean age: 55 years) | Length: Acute (single consumption) Study design: Cross-over trial Treatment: 500 g of cladode given to fasted (12 h) subjects. Cladode prepared as broiled, blended, crude, and heated (60 °C) crude. Measurements: GLU at 40, 60, 120, 180 min following intervention. |
Reductions in GLU ( | p | < 0.01) reached at 120 and 180 min. Major hypoglycemic effects shown after cladode consumption ranged from 23.3 ± 4.4 to 25.4 ± 14.3 mg/dL. No difference in the hypoglycemic effects between cladode preparations (all | p | > 0.05). | |||||||||||||||||||||||
| Frati et al. | [38] | To investigate the effect of | Opuntia streptacantha | cladode on hyperglycemia in T2DM subjects. |
Group 1: T2DM participants ( | n | = 16; 9 M and 7 F; mean age: 43.8 ± 11.4 years) Group 2: T2DM participants ( | n | = 10; 6 M and 4 F; mean age: 46.2 ± 10.8 years) Group 3: T2DM participants ( | n | = 6; 4 M and 2 F; mean age: 48.0 ± 11.7) | Length: Acute (single consumption) Study design: Randomized control-trial Treatment (fasted 12 h): Group 1: 500 g of broiled cladode. Group 2: 400 mL of water. Group 3: 500 g of broiled cladode (test 1), 400 mL of water (test 2), 500 g of broiled squash (zucchini) (test 3) Measurements: GLU at 0, 60, 120, 180 min following intervention. |
Group 1: Reduction in GLU ( | p | < 0.001) with mean reduction of 17.6 ± 2.2% of basal value at 180 min. Group 2: No change in GLU ( | p | > 0.05). Group 3: Test 1—reduction in GLU ( | p | < 0.001) with mean reduction of 16.2 ± 1.8% of basal value at 180 min; test 2, 3—no change in GLU ( | p | > 0.05). | |||||||||||||||
| Frati et al. | [13] | To evaluate the acute hypoglycemic effect of | O. streptacantha Lem. | intake in “healthy” and diabetic individuals. | Group 1: T2DM participants ( | n | = 14; 9 M and 5 F; mean age: 43 years; age range: 36–65 years). Group 2: “Healthy” participants ( | n | = 14; 9 MJ and 5 F; mean age: 33 years; age range: 15–45 years) | Length: Acute (single consumption) Study design: Randomized control trial Group 1 and 2 treatments: 500 g steamed cladode or 400 mL water (placebo) given to fasted subjects. Measurements: GLU, INS at 0, 60, 120, and 180 min following the intervention. |
Group 1: Reduction in GLU (60 min: | p | < 0.005; 120 min: | p | < 0.005; 180 min: | p | < 0.005) reaching 40.8 + 4.6 mg/dL less than basal value. Reduction in INS (120 min: | p | < 0.005; 180 min: | p | < 0.005) reaching 7.8 + 1.5 µU/mL less than basal value. Group 2: No change in GLU and INS ( | p | > 0.05). | |||||||||||||
| Frati et al. | [15] | To assess the acute hypoglycemic effect of | O. streptacantha Lem. | intake in “healthy” adults. | “Healthy” participants ( | n | = 16) Group 1: ( | n | = 5) Group 2: ( | n | = 6) Group 3: ( | n | = 5) | Length: Acute (single consumption) Group 1: 12 hr fasted + 100 g of cladode Group 2: OGTT (25 g GLU load), 100 g of cladode given after time 0, before GLU load. Group 3: OGTT (25 GLU load) + 100 g of cladode Measurements: GLU, INS at 0, 30, 60, 120 and 180 min following intervention. |
Group 1: Attenuation of GLU at 60 min; 180 min ( | p | < 0.025). No change in INS ( | p | > 0.05). Group 2, 3: No change in GLU, INS ( | p | > 0.05). | |||||||||||||||
| Guevara-Cruz et al. | [16] | To investigate the effect of dietary patterns, featuring nopal cladode, on biochemical markers (GLU, INS). | MetS participants ( | n | = 67; age: 20–60 years; satisfied 3 positive criteria for MetS). | 2 weeks prior to treatment: Participants were put on a reduced energy diet, low saturated fat, and low cholesterol diet (50–60% CH, 15% PRO and 25–35% fat). Treatment: Length: 2 months Study design: Single-center, randomized, placebo-controlled, double-blind, parallel-arm study. Group 1: Controlled dietary pattern Group 2: Placebo Dietary pattern: 100 g of cladode, 4 g of chia seeds, 22 g of oats, 32 g of soybean proteins, 0.02 g of sweetener, and 1 g of flavoring. Placebo: 30 g of calcium caseinate, 30 g of maltodextrin, 0.02 g of sweetener and 1 g of flavoring. Pre/post measurements: GLU, INS. |
Group 1: Reductions in GLU AUC (from 388.8 ± 115.2 mg/dL to 351.0 ± 115.2 mg/dL), and in AUC INS (from 26.4 ± 14.4 ng/mL to 17.4 ± 10.4 ng/mL) ( | p | < 0.0001). Group 2: No difference in GLU, INS ( | p | > 0.05). | |||||||||||||||||||||||||
| Linarès et al. | [39] | The study aimed to evaluate “NeOpuntia” on blood lipid parameters and MetS, including glycemia |
MetS participants ( | n | = 59; 0 M and 59 F; age distribution: 10.29% <35, 27.94% 35 to 45, 41.18% 45 to 55 and 29.59% >55; mean age: 47.3 ± 10.1 years) Group 1: Treatment ( | n | = 35) Group 2: Placebo ( | n | = 33) | Length: 6-weeks Study design: Monocentric, randomized, double-blind, placebo-controlled study Group 1: balanced diet (45% CH, 17% PRO and 38% fats; 2000 kcal), 3 x “NeOpuntia” capsule after meals/day. Group 2: balanced diet (45% CH, 17% PRO and 38% fats; 2000 kcal), 3 x placebo capsule after meals/day. Measurements: GLU at day 1, day 14 and day 42. |
Group 1: Treatment group remained at the same GLU level. Group 2: Increase in GLU. |
|||||||||||||||||||||||||
| Godard et al. | [40] | To assess the acute and hypoglycemic effect of OpunDia™ ( | O. ficus indica | ) in obese and pre-diabetic individuals. | Pre-diabetic and obese participants ( | n | = 29; age: 20–50 years) Group 1: Treatment ( | n | = 15) Group 2: Placebo ( | n | = 14) | Length: Acute phase (single consumption) and chronic phase (16-weeks) Acute phase: Group 1: 400 mg bolus of OpunDia™ 30 min before OGGT (75 g GLU load). Group 2: 400 mg of the placebo 30 min before OGGT (75 g GLU load). Pre/post measurements: GLU Chronic phase: Group 1: 16-week supply of 200 mg OpunDia™ Group 2: 16-week supply of the placebo Pre/post measurements: GLU |
Acute phase: Reductions in GLU in the treatment compared to placebo at 60 (205.92 ± 36.90 and 188.84 ± 38.43 mg/dL respectively), 90 (184.55 ± 33.67 and 169.74 ± 35.16 mg/dL respectively) and 120 min (159.24 ± 17.85 and 148.89 ± 24.86 mg/dL respectively) ( | p | < 0.05). Chronic phase: No difference in GLU ( | p | > 0.05) | |||||||||||||||||||
| López-Romero et al. | [41] | To investiage the effect of nopal in breakfast (2 compositions) upon metabolic markers in T2DM and “healthy” individuals | Study 1: “healthy” participants ( | n | = 4; 3 M and 4 F; mean age: 20.6.3 ± 1.2 years; mean BMI: 23.05 ± 0.8). Study 2: T2DM participants ( | n | = 14; 4 M and 10 F; mean age: 48.0 ± 2.1; mean BMI: 28.9 ± 1.0; glycosylated hemoglobin levels mean: 6.5 ± 0.2%) | Study 1: Length: Acute (single consumption) Group 1 (treatment): 50 g of dehydrated nopal. Group 2 (placebo): 50 g of available carbohydrates from GLU. Study 2: Length: Acute (single consumption) Group 1 (treatment): High CH breakfast (HCB) or high soy-protein breakfast (HSBP) with or without (random) 300 g steam nopal. Group 2 (placebo): HCB or HSBP. HCB: 300 kcal, 89% CH, 6% PRO, 5% fat in apple juice (240 mL), white bread (55.6 g) and strawberry jam (21 g). HSP: 344 kcal, 42.4% CH, 40.7% PRO, 16.9% fat in soy hamburger (61.5 g) and soymilk beverage (230 mL) Pre/post measurements: GLU, Glycemic index, insulinemic index, glucagon-like peptide 1 (GIP-1) index. |
Study 1: Glycemic index is 32.5 ± 4.0, insulinemic, Gastric Inhibitory Polypeptide index 6.5 ± 3.0, and GLP-1 index was 25.9 ± 18.0. Study 2: Group 1: Reduction in GLU AUC of HCB + nopal compared to only HCB (287 ± 30 and 443 ± 49 respectively). Reduction in GLU peaks HSPB + nopal at 30 min and 45 min ( | p | < 0.05). | |||||||||||||||||||||||||
5. Potential Hypoglycemic Mechanism of Action
Cladode consumption has been observed to exert acute hypoglycemic effects [12][13][14]; however, the exact mechanisms of its action are still not completely elucidated [12]. A study conducted by Leem et al. [42] investigated the underlying mechanism of the in vitro antidiabetic effect of Opuntia ficus indica var. saboten cladode powder extract in rats with L6 myoblasts [42]. The addition of the extract produced an increase in adenosine monophosphate-activated protein kinase (AMPK) and p38 mitogen-activated kinase (MAPK) activities (p < 0.05) within the myoblasts (Figure 2) compared to control cells (100 nM insulin). The latter enzymes are implicated as cellular energy sensors that promote the transport of glucose to skeletal muscle. The activation of AMPK promotes its interaction with p38 MAPK, which, in turn, induces the translocation of glucose 4 transporters (GLUT4) to the cell membrane [42][43]. This causes an increase in the available GLUT4 transporters on the plasma membrane, leading to an 11.7% increase in glucose uptake following the treatment when compared to a control. To confirm these findings, AMPK and p38MAPK were inhibited, which largely abolished the effects of the cladode extract treatment on the L6 myotubes’ glucose uptake. Interestingly, AMPK activation noticeably protects against hepatic lipotoxicity, a known contributor to the pathogenesis of T2DM, in cultured hepatocytes and animal liver [44].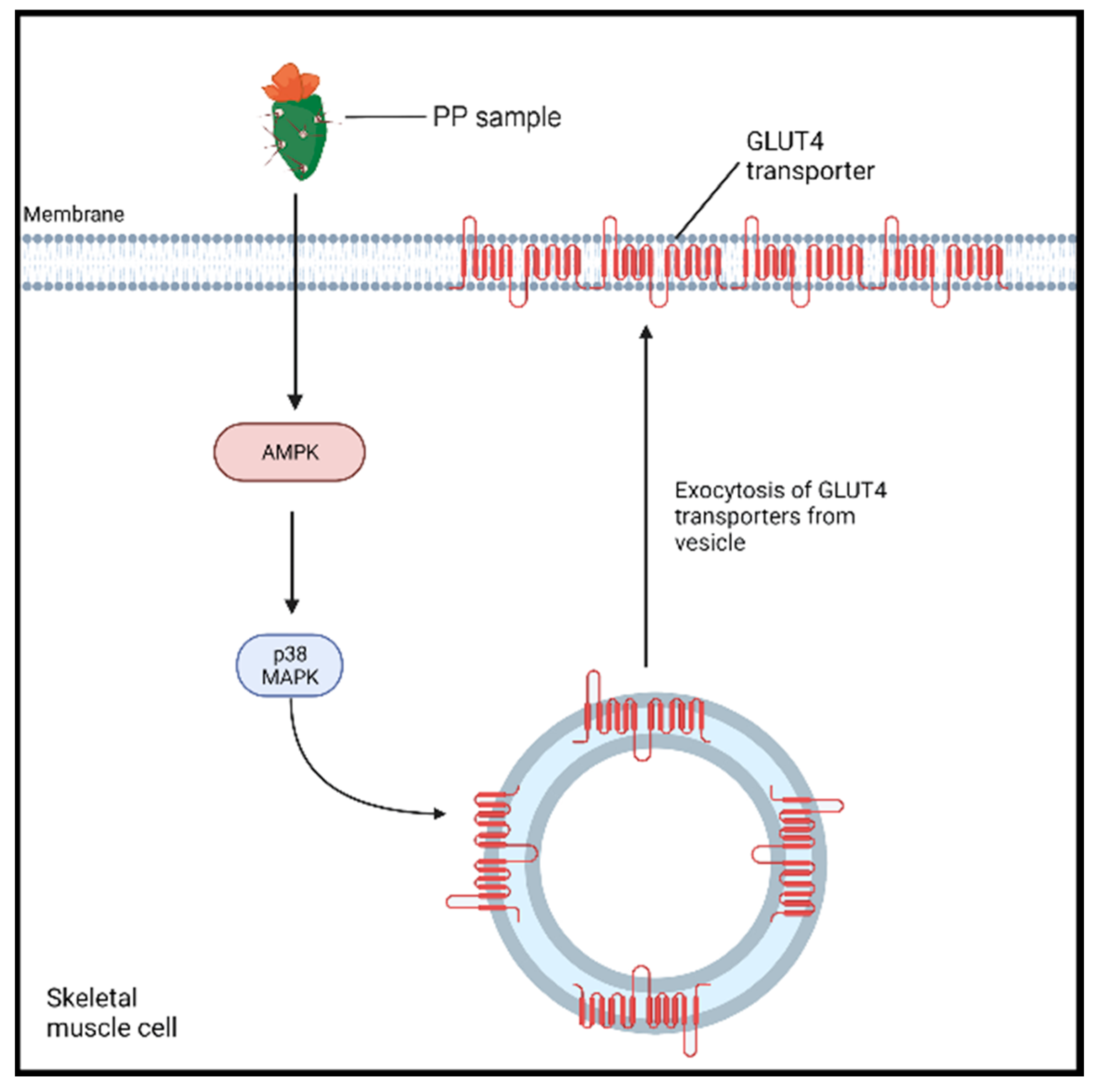
6. Prickly Pear Cladode as a Functional Ingredient for Hyperglycemia Management
7. Adverse Effects of Prickly Pear Consumption
The reported adverse effects of cladode consumption in the literature remain scarce. Nonetheless, a documented case study of low colonic obstruction in a patient was attributed to PP seed intake. Furthermore, a systematic review investigating gastrointestinal seed bezoar cases revealed that 28 individuals had phytobezoars induced by PP consumption [51]. A bezoar is a persistent indigestible mass accumulating within the gastrointestinal tract, with the most common presenting symptoms being rectal pain, intestinal obstruction, and constipation [52]. Although the occurrence of PP-induced bezoar is rare, individuals should be vigilant if any pertinent symptoms arise, especially given the association of T2DM with gastroparesis. A few anecdotal cases have been reported, with subjects presenting with chronic diarrhea and encopresis that was attributed to PP intake [53]. It would also be advisable that individuals with low blood glucose may want to abstain from PP consumption as it may exacerbate their hypoglycemia.8. Conclusions
Several studies showed reductions in blood glucose levels in individuals following the intake of cladode, particularly in an acute study design. The mechanism(s) by which the cladode exerts its hypoglycemic activity remains undefined; however, various mechanisms have been proposed. These include a cladode-induced AMPK pathway, ultimately stimulating the translocation of GLUT4 transporters to the membrane. Another mechanism by which cladode exerts its activity is by affecting the gut microbiota, ultimately altering the glucose metabolism to reduce glucose peaks. Additionally, the nopal may potentially suppress the inflammatory effect of LPS, thereby inhibiting LPS-induced insulin resistance and glucose intolerance. Given its potential anti-hyperglycemic effect, the use of PP in the production of functional foods, such as functional pasta, may represent a promising nutritional tool to manage hyperglycemia.References
- Leitner, D.R.; Fruhbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and type 2 diabetes: Two diseases with a need for combined treatment strategies—Easo can lead the way. Obes. Facts 2017, 10, 483–492.
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689.
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310.
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846.
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (dys)function and insulin resistance: From pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 2019, 10, 532.
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s disease: Mechanisms and nutritional aspects. Clin. Nutr. Res. 2018, 7, 229.
- Dyson, P.A.; Twenefour, D.; Breen, C.; Duncan, A.; Elvin, E.; Goff, L.; Hill, A.; Kalsi, P.; Marsland, N.; McArdle, P.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabetic Med. 2018, 35, 541–547.
- Gouws, C.; Mortazavi, R.; Mellor, D.; McKune, A.; Naumovski, N. The effects of prickly pear fruit and cladode (Opuntia spp.) consumption on blood lipids: A systematic review. Complement. Ther. Med. 2020, 50, 102384.
- Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Naumovski, N. Tocotrienols, health and ageing: A systematic review. Maturitas 2017, 95, 55–60.
- D’Cunha, N.M.; McKune, A.J.; Panagiotakos, D.B.; Georgousopoulou, E.N.; Thomas, J.; Mellor, D.D.; Naumovski, N. Evaluation of dietary and lifestyle changes as modifiers of S100β levels in Alzheimer’s disease. Nutr. Neurosci. 2019, 22, 1–18.
- D’Cunha, N.M.; Georgousopoulou, E.N.; Dadigamuwage, L.; Kellett, J.; Panagiotakos, D.B.; Thomas, J.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br. J. Nutr. 2018, 119, 280–298.
- Gouws, C.A.; Georgousopoulou, E.N.; Mellor, D.D.; McKune, A.; Naumovski, N. Effects of the consumption of prickly pear cacti (Opuntia spp.) and its products on blood glucose levels and insulin: A systematic review. Med. Kaunas 2019, 55, 138.
- Frati, A.C.; Gordillo, B.E.; Altamirano, P.; Ariza, C.R.; Cortés-Franco, R.; Chávez-Negrete, A.; Islas-Andrade, S. Influence of nopal intake upon fasting glycemia in type ii diabetics and healthy subjects. Arch. Investig. Med. Mex. 1991, 22, 51–56.
- Frati, A.C.; Jiménez, E.; Ariza, C.R. Hypoglycemic effect of Opuntia ficus indica in non insulin-dependent diabetes mellitus patients. Phytother. Res. 1990, 4, 195–197.
- Frati-Munari, A.C.; Yever-Garcés, A.; Islas-Andrade, S.; Ariza-Andráca, C.R.; Chávez-Negrete, A. Studies on the mechanism of “hypoglycemic” effect of nopal (Opuntia sp.). Arch. Investig. Med. Mex. 1987, 18, 7–12.
- Guevara-Cruz, M.; Tovar, A.R.; Aguilar-Salinas, C.A.; Medina-Vera, I.; Gil-Zenteno, L.; Hernandez-Viveros, I.; Lopez-Romero, P.; Ordaz-Nava, G.; Canizales-Quinteros, S.; Guillen Pineda, L.E.; et al. A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. J. Nutr. 2012, 142, 64–69.
- Trejo-Gonzalez, A.; Gabriel-Ortiz, G.; Puebla-Perez, A.M.; Huizar-Contreras, M.D.; Munguia-Mazariegos, M.R.; Mejia-Arreguin, S.; Calva, E. A purified extract from prickly pear cactus (Opuntia fuliginosa) controls experimentally induced diabetes in rats. J. Ethnopharmacol. 1996, 55, 27–33.
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951.
- Speer, H.; D’Cunha, N.M.; Davies, M.J.; McKune, A.J.; Naumovski, N. The physiological effects of amino acids arginine and citrulline: Is there a basis for development of a beverage to promote endurance performance? A narrative review of orally administered supplements. Beverages 2020, 6, 11.
- Slimen, I.B.; Najar, T.; Abderrabba, M. Opuntia ficus-indica as a source of bioactive and nutritional phytochemicals. J. Food Nutr. Sci. 2016, 4, 162.
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296.
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296.
- Javeed, N.; Matveyenko, A.V. Circadian etiology of type 2 diabetes mellitus. Physiol. Bethesda 2018, 33, 138–150.
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front. Nutr. 2018, 5, 77.
- Hotamisligil, G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. 2008, 32, S52–S54.
- Borén, J.; Taskinen, M.R.; Olofsson, S.O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40.
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory cytokines and the risk to develop type 2 diabetes. Diabetes 2003, 52, 812.
- Mayer, J.A.; Cushman, J.C. Nutritional and mineral content of prickly pear cactus: A highly water-use efficient forage, fodder and food species. J. Agron. Crop Sci. 2019, 205, 625–634.
- Shackleton, R.T.; Witt, A.B.R.; Piroris, F.M.; van Wilgen, B.W. Distribution and socio-ecological impacts of the invasive alien cactus opuntia stricta in eastern africa. Biol. Invasions 2017, 19, 2427–2441.
- Guevara, J.C.; Yahia, E.M.; Brito de la Fuente, E. Modified atmosphere packaging of prickly pear cactus stems (Opuntia spp.). LWT-Food Sci. Technol. 2001, 34, 445–451.
- Patel, S. Opuntia cladodes (nopal): Emerging functional food and dietary supplement. Mediterr. J. Nutr. Metab. 2014, 7, 11–19.
- Ramírez-Moreno, E.; Marqués, C.; Sánchez-Mata, M.C.; Goñi, I. In vitro calcium bioaccessibility in raw and cooked cladodes of prickly pear cactus (Opuntia ficus-indica L. Miller). LWT-Food Sci. Technol. 2011, 44, 1611–1615.
- Loretta, B.; Oliviero, M.; Vittorio, M.; Bojórquez-Quintal, E.; Franca, P.; Silvia, P.; Fabio, Z. Quality by design approach to optimize cladodes soluble fiber processing extraction in Opuntia ficus indica (L.) Miller. J. Food Sci. Technol. 2019, 56, 3627–3634.
- Perucini-Avendaño, M.; Nicolás-García, M.; Jiménez-Martínez, C.; Perea-Flores, M.D.J.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and structural properties, biological activity, and polyphenols profile. Food Sci. Nutr. 2021, 9, 4007–4017.
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904.
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320.
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301.
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 1–10.
- Frati-Munari, A.C.; Gordillo, B.E.; Altamirano, P.; Ariza, C.R. Hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care 1988, 11, 63–66.
- Linarès, E.; Thimonier, C.; Degre, M. The effect of neopuntia on blood lipid parameters—Risk factors for the metabolic syndrome (Syndrome X). Adv. Ther. 2007, 24, 1115–1125.
- Godard, M.P.; Ewing, B.A.; Pischel, I.; Ziegler, A.; Benedek, B.; Feistel, B. Acute blood glucose lowering effects and long-term safety of opundia™ supplementation in pre-diabetic males and females. J. Ethnopharmacol. 2010, 130, 631–634.
- Lopez-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vazquez-Manjarrez, N.; Tovar, A.R.; Pedraza-Chaverri, J.; Torres, N. The effect of nopal (Opuntia ficus indica) on postprandial blood glucose, incretins, and antioxidant activity in mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J. Acad. Nutr. Diet. 2014, 114, 1811–1818.
- Leem, K.H.; Kim, M.G.; Hahm, Y.T.; Kim, H.K. Hypoglycemic effect of Opuntia ficus-indica var. Saboten is due to enhanced peripheral glucose uptake through activation of ampk/p38 mapk pathway. Nutrients 2016, 8, 800.
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, D.G. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019, 9, 190099.
- Li, S.; Qian, Q.; Ying, N.; Lai, J.; Feng, L.; Zheng, S.; Jiang, F.; Song, Q.; Chai, H.; Dou, X. Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front. Pharmacol. 2020, 11, 1718.
- Aw, W.; Fukuda, S. Understanding the role of the gut ecosystem in diabetes mellitus. J. Diabetes Investig. 2018, 9, 5–12.
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590.
- Williams, J.; Kellett, J.; Roach, P.; McKune, A.; Mellor, D.; Thomas, J.; Naumovski, N. L-theanine as a functional food additive: Its role in disease prevention and health promotion. Beverages 2016, 2, 13.
- Contor, L. Functional food science in europe. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 20–23.
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019, 6, 94.
- Ali, A.; Rahut, D.B. Healthy foods as proxy for functional foods: Consumers’ awareness, perception, and demand for natural functional foods in pakistan. Int. J. Food Sci. 2019, 2019, 6390650.
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.H.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901.
- Sulaiman Ambusaidi, F.M.; Al-Yaqoubi, M. Gastric bezoar. Int. J. Pediatr. Adolesc. Med. 2020, 7, 199–200.
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296.
