Echocardiography, including transthoracic two and three-dimensional echocardiography, Doppler imaging, myocardial deformation and transesophageal echo, is an established and widely available imaging technique for the identification of cardiovascular manifestations that are crucial for prognosis in rheumatic diseases. Echocardiography is also important for monitoring the impact of drug treatment on cardiac function, coronary microcirculatory function, valvular function and pulmonary artery pressures.
- autoimmune rheumatic diseases
- echocardiography
- three dimensional echocardiography
- myocardial deformation
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Autoimmune rheumatic diseases (ARD) are immune-mediated diseases targeting the connective tissues. Cardiovascular complications are frequent and almost all cardiac structures may be affected. The main cardiovascular manifestations in ARD are (a) pericardial, myocardial and vascular inflammation, (b) coronary artery disease (CAD) and dysfunction of the coronary microcirculation, (c) structural and functional abnormalities of the heart valves and (d) elevated pulmonary artery pressures [1,2][1][2].
Anti-inflammatory treatment reduces inflammation and disease-associated morbidity and mortality. However, the incidence of cardiovascular (CV) events is higher in ARD patients in comparison with the general population resulting in worse prognosis [3]; thus, the early identification of cardiovascular abnormalities is crucial in order to improve outcomes.
Echocardiography is an established and widely available imaging technique for the detection of cardiovascular involvement and for monitoring the effects of treatment on cardiac and vascular structure and function in ARD patients. In this article, we aim to review the role of echocardiography for diagnosis and prognosis in ARD associated with increased incidence of cardiovascular complications and higher cardiovascular risk [4] [4] including rheumatoid arthritis (RA) systemic lupus erythematosus (SLE), systemic sclerosis (SSc), psoriasis and psoriatic arthritis and ankylosing spondylitis (AS).
2. Echocardiographic Assessment of Cardiovascular Involvement in Autoimmune Rheumatic Diseases
The main echocardiographic findings consistent with cardiovascular involvement in autoimmune rheumatic diseases are summarized in Table 1.
Table 1. Main echocardiographic findings consistent with cardiovascular involvement in autoimmune rheumatic diseases.
| Cardiovascular Manifestations | Abnormalities Consistent with Cardiovascular Involvement | Echocardiographic Parameters for Diagnosis and Assessment of Severity |
|---|---|---|
| Pericarditis | Pericardial effusion | Loculated or circumferential. Mild >10 mm, moderate 10–20 mm, large >20 mm |
| Tamponade | Early RV diastolic collapse, late RA diastolic collapse, swinging heart, respiratory variation in ventricular chamber size, dilated inferior vena cava. Exaggerated respiratory changes of >25% in mitral inflow and aortic outflow velocity. Respiratory variation of the mitral peak E velocity of >25% | |
| Constrictive pericarditis | Septal bounce, pericardial thickening. Preserved Tissue Doppler e’ velocity >8.0 cm/s | |
| Myocarditis, ischemic cardiomyopathy | Impaired LV systolic function | Wall motion abnormalities, impaired LVEF |
| LV diastolic dysfunction | LA volume index >34 mL/m2. In patients with normal EF >50%, ratio E/e’ >14, Tissue Doppler e’ velocity of the interventricular septum >7 cm/s or Tissue Doppler e’ velocity of the lateral wall >10 cm/s, TRVmax >2.8 m/s | |
| Impaired RV systolic function | TAPSE >17 mm, FAC >35%, Impaired RVEF (3D echo). S’RV >9.5 cm/s | |
| Valvular heart disease | Valvular abnormalities | Valve thickening, prolapse of mitral leaflets, valvular nodules in RA, Libman–Sacks vegetations in SLE, Libman–Sacks like vegetations in RA. Moderate or severe valvular regurgitation, rarely stenosis |
| Pulmonary hypertension | Dilation of right chambers, ventricular interdependence | RV/LV >1 diameter ratio, flattened interventricular septum, dilated pulmonary artery diameter >25 mm, right atrial area >18 cm2, dilated inferior vena cava >21 mm with reduced inspiratory collapse. TRVmax >2.8 m/s and presence of secondary signs suggestive of PH: RV outflow velocity acceleration time >105 m/s, early diastolic pulmonary regurgitation velocity >2.2 m/s |
| Aortitis | Thickening of aortic walls | |
| Subclinical LV dysfunction Subclinical RV dysfunction | Impaired GLS | GLS >−20% |
| Impaired RV longitudinal strain | RV free wall Longitudinal Strain >−20% | |
| Obstructive epicardial CAD and/or coronary microcirculation dysfunction | New wall motion abnormalities during stress echo | |
| Impaired coronary flow reserve | Coronary Flow Reserve >2 |
3. Pericardial Involvement
In case of suspected pericarditis in ARD, the amount and location of pericardial fluid and the rare presence of tamponade and constrictive pericarditis can be assessed with two-dimensional echocardiography (2D echo) and Doppler imaging [28,29][5][6]. Swinging heart, early right ventricular diastolic collapse, late right atrial diastolic collapse, respiratory variation in ventricular chamber size, dilated inferior vena cava and exaggerated mitral inflow respiratory variability of >25% are findings consistent with cardiac tamponade [29][6]. Septal bounce exaggerated respiratory changes of the mitral E wave and Tissue Doppler mitral e’ velocity >8.0 cm/s [29] [6] are findings indicative of constrictive pericarditis. However, the extent and location of pericardial thickening and calcification often cannot be reliably assessed with 2D echo. In such cases, cardiac CT can be used to detect pericardial thickening and the degree of the fibrocalcific process as well as the amount and location of pericardial fluid in challenging echo windows [29][6].
4. Myocardial Involvement
4.1. Rheumatoid Arthritis
As some studies have indicated that the systolic function of the LV may be moderately reduced in RA patients suggesting progression to heart failure [30][7], assessment of LV performance in relevance with the occurrence of symptoms of heart failure is very important. Global hypokinesis or segmental wall motion abnormalities can be detected with 2D echo, whereas assessment of diastolic function and LV filling pressures with Doppler echocardiography are also crucial [31,32][8][9]. In patients with normal ejection fraction (EF), a ratio of E/e’ >14, tissue Doppler e’ velocity of the interventricular septum <7 cm/s or tissue Doppler e’ velocity of the lateral wall >10 cm/s, left atrial volume index >34 mL/m2 and tricuspid regurgitation maximum velocity >2.8 m/s are findings consistent with elevated filling pressures [32][9]. Myocardial dysfunction has been associated with high disease activity and duration in RA [33,34][10][11].
Although extensively validated and used in daily practice, left ventricular ejection fraction (LVEF) is not the ideal marker for the evaluation of myocardial function because a reduction in LVEF is indicative of a relatively advanced myocardial dysfunction. Speckle tracking deformation analysis is considered a more sensitive technique for the identification of subclinical alterations in myocardial contractility, providing a more comprehensive study of myocardial function [35][12].
It has been demonstrated that subclinical myocardial dysfunction may be present in very early RA patients without CV risk factors. Impaired values of global longitudinal and circumferential strain were observed in RA patients with preserved ejection fraction and high disease activity compared with healthy controls [36,37][13][14].
Inflammatory cytokines including tumor necrosis-α (TNFα), interleukin-1 (IL-1) and interleukin-6 (IL-6) have a pivotal role in RA and may induce myocardial and vascular dysfunction and promote LV remodeling and fibrosis [38,39,40][15][16][17].
Speckle tracking deformation analysis can be applied in ARD patients with unexplained dyspnea and a preserved ejection fraction. Impaired global longitudinal strain (GLS) values in combination with echocardiography findings of elevated filling pressures in the absence of valvular disease or pulmonary abnormalities are consistent with heart failure with preserved ejection fraction [32,35][9][12]. Speckle tracking is also useful for the assessment of the effects of anti-inflammatory treatment in RA. It has been shown that RA patients compared with normal controls had impaired longitudinal (−18.5% vs. −22.50%, respectively), circumferential and radial strain [41][18]. Impaired longitudinal and circumferential strain values correlated with increasing values of inflammation markers (interleukin-1β) and markers of oxidative stress (protein carbonyl). In patients with RA and coexisting CAD, LVEF was lower compared with normal controls and RA patients without CAD. Lower LVEF values correlated with elevated values of oxidative stress markers (protein carbonyl, nitrotyrosine and malondialdehyde) [42][19].
Treatment with an interleukin-1 (IL-1) inhibitor (anakinra) resulted in improved LV global longitudinal strain (Figure 1).
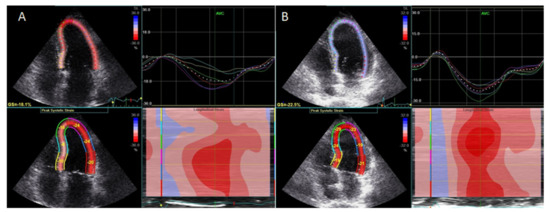
LV twisting-untwisting in RA patients, in parallel with a reduction in oxidative stress markers and interleukin-6, particularly in patients with coexisting CAD [40[17][20][19][21],41,42,43], suggested improvement of myocardial function with targeted anti-inflammatory treatment. Finally, treatment with an IL-6 inhibitor (tocilizumab) resulted in a greater improvement of GLS and reduction in markers of inflammation C-reactive protein (CRP) and oxidative stress (protein carbonyl and malondialdehyde), compared with conventional synthetic disease-modifying antirheumatic drugs or glucocorticoids [44][22].
The impairment of circumferential systolic and diastolic strain has been associated with disease activity and the degree of inflammation and diffuse fibrosis as expressed by expansion of extracellular volume by cardiac magnetic resonance (CMR) T1 mapping [45,46][23][24].
Finally, LV hypertrophy is associated with an increased prevalence of cardiovascular events in the general population and in RA patients. TNFα seems to be a significant determinant of LV remodeling [47][25]. It has been demonstrated that treatment with the TNFα inhibitor etanercept may significantly reduce LV hypertrophy [48] [26] and TNFα inhibition may improve longitudinal strain values in RA patients [37][14], suggesting the reversal of myocardial structural and functional abnormalities with anti-inflammatory treatments.
4.2. Systemic Lupus Erythematosus
Patients with SLE myocarditis may have global or segmental wall motion abnormalities by 2D echo and low systolic Tissue Doppler imaging velocities of the mitral annulus [6,49][27][28]. Impaired biventricular myocardial strain by speckle tracking has also been reported to be suggestive of subclinical myocardial dysfunction [50,51][29][30]. Impaired LV longitudinal strain values correlated with a more advanced degree of diffuse fibrosis by CMR T1 mapping [52][31].
4.3. Systemic Sclerosis
Transthoracic 2D echo is a valuable tool for the assessment of heart function in SSc, whereas 3D echo may offer additional information on ventricular volumes and EF especially in case of PAH affecting the right ventricle (RV). Structural and functional abnormalities of the LV and RV have been reported in up to 23% and 21% of SSc patients, respectively [53][32]. Primary SSc involvement of the myocardium typically affects the LV, leading to reduced LVEF (in 5.4%), LV hypertrophy (in 22.6%) and LV diastolic dysfunction (in 17.7%) [54,55][33][34]. Tissue-Doppler imaging and speckle tracking may detect subtle myocardial dysfunction in SSc [56,57][35][36].
Patients with SSc and preserved EF as compared with controls had impaired GLS (−18.2 ± 1.8% vs. −21.3 ± 1.7%, respectively (p > 0.01)) and circumferential strain values (−18.2 ± 2.3% vs. −21.3 ± 2.1%, respectively (p > 0.01)). In patients with SSc, impaired GLS and circumferential strain values each correlated with worse functional capacity and rhythm disturbances [57][36].
It has also been demonstrated that global longitudinal strain of the right ventricle is impaired in SSc patients compared with age and sex-matched controls (−17.7% vs. −20.4%, respectively) [58][37].
Impaired myocardial strain correlated with disease activity and the amount of focal and diffuse myocardial fibrosis by CMR T1 mapping [59] [38] and has been associated with increased cardiovascular risk in SSc [60][39].
4.4. Psoriasis
It has been demonstrated that GLS is impaired in a similar degree in psoriasis and CAD patients compared with normal controls (−16.5% in psoriasis vs. −16.2% in CAD vs. −21.9% in controls). This is consistent with the presence of subclinical myocardial dysfunction in psoriasis due to a similarly elevated inflammatory and oxidative stress burden as indicated by elevated IL-6 and malondialdehyde values [26][40]. Treatment with an interleukin-12/23 inhibitor resulted in a greater improvement of GLS compared with treatment with a TNFα inhibitor or cyclosporine, in parallel with a reduction of IL-6, IL-12, IL-17, TNFα and malondialdehyde levels [61][41]. Moreover, the magnitude of GLS improvement correlated with the reduction in oxidative stress and inflammatory markers (IL-6, IL-12 and malondialdehyde). Treatment with interleukin-17 inhibitors also resulted in a greater improvement of systolic and diastolic longitudinal myocardial deformation markers and LV twist compared with cyclosporine or methotrexate treatment. Changes in myocardial deformation markers correlated with the degree of reduction of oxidative stress markers (malondialdehyde and protein carbonyl levels) [62][42].
5. Valvular Heart Disease
5.1. Rheumatoid Arthritis
Two and three dimensional transesophageal echocardiography is an accurate technique for the diagnosis and assessment of the severity of valvular heart disease [1,7,63][43][44][45]. Rheumatic valve nodules are detected in 32% of RA patients [7]. Valve nodules are oval shaped and small in size (4–12 mm) formations. Aortic and mitral valves are equally affected. Rheumatic nodules have homogenous echoreflectancy and regular borders and they are located at the leaflets’ basal or mid segments, the myocardium or chordae tendinae [7][44]. Moderate to severe valve regurgitation may be detected due to the thickening and prolapse of the mitral leaflets in the case of RA valvulitis [1][43]. Libman–Sacks-like vegetations are rarely observed [1][43].
5.2. Systemic Lupus Erythematosus
Valvular heart disease may be identified in 30–50% with transthoracic and 60–70% with transesophageal echocardiography in SLE patients [1][43]. Valvular disease is characterized by the thickening of the mid portions and the tips of the leaflets. Leaflet prolapse or perforation, chordal rupture and significant valvular regurgitation may be detected, whereas valve stenosis is rare [1][43].
Libman–Sacks vegetations are detected in 10% by transthoracic and 30–40% by transesophageal echocardiography in SLE patients. Libman–Sacks vegetations are typically located on the tips of the left heart valves as inflammatory formations of >2 mm in diameter, or thrombotic or firm masses that may resolve or relapse [1][43]. Thrombus formation on the vegetations may lead to macro or microembolic events [1][43]. Compared with mild valvular abnormalities, moderate to severe valvular dysfunction was associated with a 3–4 times higher rate of occurrence of symptoms of valvular disease, the need for valve surgery, stroke or other embolic events and death during a follow-up period of 2–8 years [1][43]. The prevalence of valvular abnormalities and other sources of embolism has been reported in up to 61% of patients with antiphospholipid syndrome with higher rates of thromboembolic events associated with higher anticardiolipin antibody titers of >40 GPL units [64][46]. Thus, patients with antiphospholipid syndrome and clinical symptoms of peripheral embolism or high anticardiolipin antibody titers should be examined with transoesophageal echo (TOE) [65][47].
5.3. Ankylosing Spondylitis
Aortitis is a frequent and serious complication of AS, occurring late in the course of the disease. The inflammatory process begins from the aortic root wall and may expand to the aortic cusps causing thickening and fibrosis [66][48]. Mitral valve and heart conduction systems may also be involved in the course of the disease [66][48]. The main echocardiography findings in the case of aortic involvement included aortic root thickening and dilatation and aortic cusp thickening [67], whereas aortic regurgitation has been reported in up to 34% of patients, mitral regurgitation in 1–76% and mitral valve prolapse in 5.7–10% [25,27].[49], whereas aortic regurgitation has been reported in up to 34% of patients, mitral regurgitation in 1–76% and mitral valve prolapse in 5.7–10% [50][51].
6. Coronary Artery Disease
6.1. Rheumatoid Arthritis
RA is characterized by a higher incidence of CAD and cardiovascular events compared with the general population due to systemic inflammation and the high prevalence of traditional CV risk factors [11,68][52][53]. Patients with RA and extra-articular symptoms, rheumatoid factors-positive, extended disease duration, additional risk factors and the presence of carotid plaques by carotid ultrasonography are at increased CV risk; thus, they should be screened for CAD [69,70][54][55]. According to European League Against Rheumatism (EULAR) recommendations, the assessment of CV risk is recommended for all patients with RA and ARD at least once every five years and after major changes in antirheumatic therapy [69][54]. Non-invasive imaging techniques may have a key role for the evaluation of CV risk in ARD patients [70][55]. A multimarker approach including various imaging modalities has been proposed for the identification of ARD patients with subclinical atherosclerosis, permitting initiation of cardiovascular protective therapies (e.g., statins) to reduce CV risk [71,72][56][57].
The detection of carotid atherosclerosis has been shown to reclassify patients with RA into a higher risk group [73][58]. In ARD patients with symptoms of CAD, stress echocardiography may offer valuable information for the diagnosis and prognostic assessment of CAD [74][59]. It has a similar diagnostic accuracy compared with radionuclide stress tests; however, it lacks radiation exposure [74][59]. It has been indicated that patients with RA had a 2-fold higher rate of positive exercise echo for myocardial ischemia compared with controls and a positive stress echo was associated with increased disease duration. Moreover, 5-year all-cause mortality was 14.9% in RA patients with a positive stress echo compared with 4.3% in those with a negative stress echo for ischemia [75][60]. Additionally, silent myocardial ischemia in the absence of obstructive coronary lesions may be detected with stress echo in RA in a similar prevalence as in diabetes mellitus due to abnormal microcirculatory function [76][61].
Coronary flow reserve can be calculated non-invasively by Doppler echocardiography of the left anterior descending artery as the ratio of peak diastolic velocity after adenosine infusion to peak diastolic velocity at rest [77][62]. Coronary flow reserve may provide information regarding the integrity of coronary microcirculation and the presence of obstructive coronary stenosis and is associated with a worse prognosis even in the absence of obstructive CAD [78][63]. A CFR value of >2 is consistent with significant epicardial coronary stenosis [79][64]. It has been demonstrated that the CFR is impaired in systemic rheumatic diseases compared with healthy controls [80][65]. Significantly lower CFR values have been found in untreated patients with early RA due to abnormalities of the coronary microcirculation [81] [66] (Figure 2).
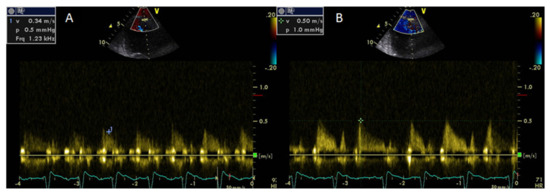
Decreasing CFR values correlated with elevated values of symmetric dimethylarginine, a marker of endothelial dysfunction that acts as an endogenous inhibitor of nitric oxide synthase [81][66].
Treatment with an interleukin-1 (IL-1) inhibitor (anakinra) resulted in improved CFR in RA patients, particularly when CAD coexisted, indicating a beneficial effect of the anti-inflammatory treatment on coronary microcirculatory function in RA [40,41,42,43][17][20][19][21].
6.2. Systemic Lupus Erythematosus
The calculation of CV risk based on the traditional risk scores is inaccurate in SLE [82][67]. Systemic inflammation and increased disease activity have a major role for the high incidence of atherosclerosis and CAD in SLE [17,83][68][69]. Atheromatic plaques were detected in 40% of SLE patients by carotid ultrasonography and plaque progression was greater in SLE patients compared with the general population [84][70]. Coronary flow reserve has been found to be impaired in young SLE patients without risk factors for CAD suggesting coronary microcirculatory dysfunction [85][71].
6.3. Psoriasis
Coronary flow reserve was similarly impaired in psoriasis and CAD patients after adjustment for atherosclerotic risk factors in parallel with elevated markers of inflammation and oxidative stress [26][40]. Moreover, treatment with IL-12/23 and IL-17 inhibitors resulted in a significant improvement of CFR, greater compared with TNFα inhibition, cyclosporine or methotrexate treatment. CFR improvement correlated with a concomitant reduction in the inflammatory and oxidative stress markers [61,62][72][73].
6.4. Ankylosing Spondylitis
Several studies have indicated an increased CV risk and accelerated atherosclerosis in AS [86,87][74][75]. A meta-analysis demonstrated a significantly increased risk for CAD in patients with AS with an 41% excess risk [88][76].
Inflammatory cytokines, oxidative stress, traditional CV risk factors and the toxic effects of non-steroid inflammatory drugs have been considered to cause aggravation of the atherosclerotic process in AS [89,90,91][77][78][79]. Echocardiography assessment of structural and functional abnormalities and especially stress echocardiography for detection of ischemia may provide significant information for risk stratification and clinical decision making in AS patients.
7. Pulmonary Hypertension
Transthoracic echocardiography is a first line modality for PAH screening and for the detection of RV dysfunction because RV remodeling has a major role in the prognosis of PAH patients [20][80]. Continuous wave Doppler measurement of TRVmax may reveal increased pulmonary artery systolic pressure. Moreover, indirect findings consistent with PAH such dilation and dysfunction of the right ventricle, systolic flattening of the interventricular septum, dilated inferior vena cava and increased pulmonary regurgitation early diastolic velocity may raise suspicion for the presence of PAH [20][80]. Exercise stress echo may identify patients at an increased risk to develop PAH. Patients with SSc, SLE or mixed connective tissue disease and a greater rise in mean pulmonary artery pressure during exercise had increased rates of PAH development in the future [92][81].
Moreover, poor contractile reserve and impaired GLS during exercise stress echo in SSc patients has been associated with reduced functional capacity and higher values of pulmonary artery systolic pressures [93][82].
Due to the complex triangular-crescent shape of the RV, a structural and functional assessment of the RV by 2D echo is based on geometrical assumptions [31][83]. 3D echo by reconstruction of the whole RV overcomes geometrical limitations for the estimation of RV volumes and right ventricular ejection fraction (RVEF). Although RV volumes are slightly underestimated by 3D echo compared with CMR, a good correlation between these two modalities has been reported [94][84].
8. Conclusions
Patients with ARD can have excess CV risk and increased incidence of CV complications. Echocardiography is the first line imaging technique for the detection of cardiovascular involvement and for monitoring the effects of treatment in ARD. Additionally, 3D echo may have an added value in volumetric evaluation especially of the right ventricle, whereas speckle tracking may accurately identify subclinical cardiac dysfunction. It remains to be elucidated in future studies whether the improvement of echo markers of myocardial deformation and microcirculatory function by reduction of the inflammatory burden will reduce adverse cardiac events and improve the prognosis of ARD patients.
References
- Roldan:, C.A. Valvular and coronary heart disease in systemic inflammatory diseases. Heart 2008, 94, 1089–1101.
- Knockaert, D.C. Cardiac involvement in systemic inflammatory diseases. Eur. Heart J. 2007, 28, 1797–1804.
- Ivana Hollan; Pier Luigi Meroni; Joseph M. Ahearn; J.W. Cohen Tervaert; Sam Curran; Carl S Goodyear; Knut A. Hestad; Bashar Kahaleh; Marcello Riggio; Kelly Shields; et al.Mary C. Wasko Cardiovascular disease in autoimmune rheumatic diseases. Autoimmunity Reviews 2013, 12, 1004-1015, 10.1016/j.autrev.2013.03.013.
- R Agca; S C Heslinga; S Rollefstad; M Heslinga; I B McInnes; M J L Peters; T K Kvien; M Dougados; H Radner; F Atzeni; et al.Jette PrimdahlA SödergrenS Wallberg JonssonJ Van RompayC ZabalanTina Ravn PedersenL JacobssonK De VlamM A Gonzalez-GayA G SembG D KitasY M SmuldersZ SzekaneczN SattarD. P. M. SymmonsMichael T. Nurmohamed EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Annals of the Rheumatic Diseases 2016, 76, 17-28, 10.1136/annrheumdis-2016-209775.
- Maksimovic, R.; Seferovic, P.M.; Ristic, A.D.; Vujisić-Tesić, B.; Simeunović, D.S.; Radovanović, G.; Matucci-Cerinic, M.; Maisch, B. Cardiac imaging in rheumatic diseases. Rheumatology 2006, 45 (Suppl. S4), iv26–iv31.
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964.
- Jon T. Giles; Ashkan A. Malayeri; Veronica Fernandes; Wendy Post; Roger S. Blumenthal; David A. Bluemke; Jens Vogel-Claussen; Moyses Szklo; Michelle Petri; Allan C. Gelber; et al.Lyndia BrumbackJoão LimaJoan M Bathon Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Care & Research 2010, 62, 940-951, 10.1002/art.27349.
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: The update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the american society of echocardiography and the european association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314.
- Montecucco, C.; Gobbi, G.; Perlini, S.; Rossi, S.; Grandi, A.M.; Caporali, R.; Finardi, G. Impaired diastolic function in active rheumatoid arthritis. Relationship with disease duration. Clin. Exp. Rheumatol. 1999, 17, 407–412.
- Hanvivadhanakul, P.; Buakhamsri, A. Disease activity is associated with LV dysfunction in rheumatoid arthritis patients without clinical cardiovascular disease. Adv. Rheumatol. 2019, 59, 56.
- Alberto Lo Gullo; Javier Rodríguez-Carrio; Romina Gallizzi; Egidio Imbalzano; Giovanni Squadrito; Giuseppe Mandraffino; Speckle tracking echocardiography as a new diagnostic tool for an assessment of cardiovascular disease in rheumatic patients.. Progress in Cardiovascular Diseases 2020, 63, 327-340, 10.1016/j.pcad.2020.03.005.
- Lo Gullo, A.; Rodríguez-Carrio, J.; Aragona, C.A.; Dattilo, G.; Zito, C.; Suárez, A.; Loddo, S.; Atteritano, M.; Saitta, A.; Mandraffino, G. Subclinical impairment of myocardial and endothelial functionality in very early psoriatic and rheumatoid arthritis patients: Association with vitamin D and inflammation. Atherosclerosis 2018, 271, 214–222.
- Atzeni, F.; Gianturco, L.; Boccassini, L.; Sarzi-Puttini, P.; Bonitta, G.; Turiel, M. Noninvasive imaging methods for evaluating cardiovascular involvement in patients with rheumatoid arthritis before and after anti-TNF drug treatment. Future Sci. OA 2019, 5, FSO396.
- Sarzi-Puttini, P.; Atzeni, F.; Shoenfeld, Y.; Ferraccioli, G. TNF-α, rheumatoid arthritis, and heart failure: A rheumatological dilemma. Autoimmun. Rev. 2005, 4, 153–161.
- Fontes, J.A.; Rose, N.R.; Cihakova, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68.
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Andreadou, I.; Triantafyllidi, H.; Tsoumani, M.; Varoudi, M.; Vlastos, D.; Makavos, G.; Kostelli, G.; et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin. Res. Cardiol. 2019, 108, 1093–1101.
- I Ikonomidis; S Tzortzis; J Lekakis; I Paraskevaidis; I Andreadou; M Nikolaou; T Kaplanoglou; P Katsimbri; G Skarantavos; P Soucacos; et al.D T. Kremastinos Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart 2009, 95, 1502-1507, 10.1136/hrt.2009.168971.
- Ikonomidis, I.; Tzortzis, S.; Andreadou, I.; Paraskevaidis, I.; Katseli, C.; Katsimbri, P.; Pavlidis, G.; Parissis, J.; Kremastinos, D.; Anastasiou-Nana, M.; et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ. Cardiovasc. Imaging 2014, 7, 619–628.
- Ikonomidis, I.; Tzortzis, S.; Lekakis, J.; Paraskevaidis, I.; Andreadou, I.; Nikolaou, M.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.; et al. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart 2009, 95, 1502–1507.
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.; Kremastinos, D.T. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008, 117, 2662–2669.
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694.
- Ntusi, N.A.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Matthews, P.M.; Robson, M.D.; Wordsworth, P.B.; Neubauer, S.D.; Karamitsos, T.D. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from cmr t1 mapping. JACC Cardiovasc. Imaging 2015, 8, 526–536.
- Holmström, M.; Koivuniemi, R.; Korpi, K.; Kaasalainen, T.; Laine, M.; Kuuliala, A.; Leirisalo-Repo, M.; Kupari, M.; Kivistö, S. Cardiac magnetic resonance imaging reveals frequent myocardial involvement and dysfunction in active rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 416–423.
- Furst, D.E.; Pangan, A.L.; Harrold, L.R.; Chang, H.; Reed, G.; Kremer, J.M.; Greenberg, J.F. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in disease course: Results from the CORRONA registry. Arthritis Care Res. 2011, 63, 856–864.
- Daïen, C.I.; Fesler, P.; Cailar, G.; Daïen, V.; Mura, T.; Dupuy, A.-M.; Cristol, J.-P.; Ribstein, L.; Combe, B.; Morel, J. Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 881–887.
- Alida L.P. Caforio; Yehuda Adler; Carlo Agostini; Yannick Allanore; Aris Anastasakis; Michael Arad; Michael Böhm; Philippe Charron; Perry M. Elliott; U. Eriksson; et al.Stephan B. FelixPablo Garcia-PaviaEric HachullaStephane HeymansMassimo ImazioKarin KlingelRenzo MarcolongoMarco Matucci-CerinicAntonis PantazisSven PleinValeria PoliAngelos RigopoulosPetar SeferovicYehuda ShoenfeldJosé L ZamoranoAleš Linhart Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. European Heart Journal 2017, 38, 2649-2662, 10.1093/eurheartj/ehx321.
- Sebastian J. Buss; David Wolf; Grigorios Korosoglou; Regina Max; Celine S. Weiss; Christian Fischer; Dieter Schellberg; Christian Zugck; H. Kuecherer; Hanns‐Martin Lorenz; et al.Hugo A. KatusStefan E. HardtAlexander Hansen Myocardial Left Ventricular Dysfunction in Patients with Systemic Lupus Erythematosus: New Insights from Tissue Doppler and Strain Imaging. The Journal of Rheumatology 2009, 37, 79-86, 10.3899/jrheum.090043.
- Poorzand, H.; Mirfeizi, S.Z.; Javanbakht, A.; Alimi, H. Comparison of echocardiographic variables between systemic lupus erythematosus patients and a control group. Arch. Cardiovasc. Imaging 2015, 3, e30009.
- Leal, G.N.; Silva, K.F.; Franca, C.M.; Lianza, A.C.; Andrade, J.L.; Campos, L.M.A.; Bonfá, E.; Silva, C.A. Subclinical right ventricle systolic dysfunction in childhood-onset systemic lupus erythematosus: Insights from two-dimensional speckle-tracking echocardiography.
- Valentina O. Puntmann; David D’Cruz; Zachary Smith; Ana Pastor; Peng Choong; Tobias Voigt; Gerry Carr-White; Shirish Sangle; Tobias Schaeffter; E. Nagel; et al. Native Myocardial T1 Mapping by Cardiovascular Magnetic Resonance Imaging in Subclinical Cardiomyopathy in Patients With Systemic Lupus Erythematosus. Circulation: Cardiovascular Imaging 2013, 6, 295-301, 10.1161/circimaging.112.000151.
- A-L Hachulla; David Launay; V Gaxotte; P. De Groote; Nicolas Lamblin; Patrick Devos; P-Y Hatron; J-P Beregi; Eric Hachulla; Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients.. Annals of the Rheumatic Diseases 2008, 68, 1878-84, 10.1136/ard.2008.095836.
- Allanore, Y.; Meune, C.; Vonk, M.C.; Airo, P.; Hachulla, E.; Caramaschi, P.; Riemekasten, G.; Cozzi, F.; Beretta, L.; Derk, C.T.; et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann. Rheum. Dis. 2010, 69, 218–222.
- de Groote, P.; Gressin, V.; Hachulla, E.; Carpentier, P.; Guillevin, L.; Kahan, A.; Cabane, J.; Francès, C.; Lamblin, N.; Diot, E.; et al. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann. Rheum. Dis. 2008, 67, 31–36.
- Meune, C.; Avouac, J.; Wahbi, K.; Cabanes, L.; Wipff, J.; Mouthon, L.; Guillevin, L.; Kahan, A.; Allanore, Y. Cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography during routine care: A controlled study of 100 consecutive patients. Arthritis Rheum. 2008, 58, 1803–1809.
- Yiu, K.H.; Schouffoer, A.A.; Marsan, N.A.; Ninaber, M.K.; Stolk, J.; Vlieland, T.V.; Scherptong, R.W.; Delgado, V.; Holman, E.R.; Tse, H.F.; et al. Left ventricular dysfunction assessed by speckle-tracking strain analysis in patients with systemic sclerosis: Relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum. 2011, 63, 3969–3978.
- Monica Mukherjee; Shang-En Chung; Von Khue Ton; Ryan J. Tedford; Laura K. Hummers; Fredrick M. Wigley; Theodore P. Abraham; Ami A. Shah; Unique Abnormalities in Right Ventricular Longitudinal Strain in Systemic Sclerosis Patients.. Circulation: Cardiovascular Imaging 2016, 9, e003792, 10.1161/CIRCIMAGING.115.003792.
- Ntobeko Ntusi; Stefan K. Piechnik; Jane Francis; Vanessa M. Ferreira; Aitzaz Bs Rai; Paul M Matthews; Matthew D. Robson; James C. Moon; Paul Wordsworth; Stefan Neubauer; et al.Theodoros D. Karamitsos Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis – a clinical study using myocardial T1-mapping and extracellular volume quantification. Journal of Cardiovascular Magnetic Resonance 2014, 16, 21-21, 10.1186/1532-429X-16-21.
- Maurizio Cusmà Piccione; Concetta Zito; Gianluca Bagnato; Giuseppe Oreto; Gianluca Di Bella; Gian Filippo Bagnato; Scipione Carerj; Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovascular Ultrasound 2013, 11, 6-6, 10.1186/1476-7120-11-6.
- Ignatios Ikonomidis; George Makavos; Evangelia Papadavid; Maria Varoudi; Ioanna Andreadou; Kostas Gravanis; Kostas Theodoropoulos; George Pavlidis; Helen Triantafyllidi Md; John Parissis; et al.Ioannis ParaskevaidisDimitrios RigopoulosJohn P. Lekakis Similarities in Coronary Function and Myocardial Deformation Between Psoriasis and Coronary Artery Disease: The Role of Oxidative Stress and Inflammation. Canadian Journal of Cardiology 2015, 31, 287-295, 10.1016/j.cjca.2014.11.002.
- Ignatios Ikonomidis; Evangelia Papadavid; George Makavos; Ioanna Andreadou; Maria Varoudi; Kostas Gravanis; Kostas Theodoropoulos; George Pavlidis; Helen Triantafyllidi Md; Paraskevi Moutsatsou; et al.Christina PanagiotouJohn ParissisEfstathios IliodromitisJohn P. LekakisDimitrios Rigopoulos Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared With Tumor Necrosis Factor-a Antagonism or Cyclosporine in Psoriasis. Circulation: Cardiovascular Imaging 2017, 10, e006283, 10.1161/circimaging.117.006283.
- George Makavos; Ignatios Ikonomidis; Ioanna Andreadou; Maria Varoudi; Irini Kapniari; Eleni Loukeri; Kostas Theodoropoulos; George Pavlidis; Helen Triantafyllidi; John Thymis; et al.John ParissisMaria TsoumaniPinelopi Rafouli-StergiouPelagia KatsimbriEvangelia Papadavid Effects of Interleukin 17A Inhibition on Myocardial Deformation and Vascular Function in Psoriasis.. Canadian Journal of Cardiology 2019, 36, 100-111, 10.1016/j.cjca.2019.06.021.
- Carlos Roldan; Valvular and coronary heart disease in systemic inflammatory diseases. Heart 2008, 94, 1089-1101, 10.1136/hrt.2007.132787.
- Eric R. Hurd; Extraarticular manifestations of rheumatoid arthritis. Seminars in Arthritis and Rheumatism 1979, 8, 151-176, 10.1016/s0049-0172(79)80005-0.
- William A. Zoghbi; David Adams; Robert O. Bonow; Maurice Enriquez-Sarano; Elyse Foster; Paul A. Grayburn; Rebecca T. Hahn; YuChi Han; Judy Hung; Roberto M. Lang; et al.Stephen H. LittleDipan J. ShahStanton ShernanPaaladinesh ThavendiranathanJames D. ThomasNeil J. Weissman Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. Journal of the American Society of Echocardiography 2017, 30, 303-371, 10.1016/j.echo.2017.01.007.
- Maurizio Turiel; Piercarlo Sarzi-Puttini; Rossana Peretti; Sara Bonizzato; Sabrina Muzzupappa; Fabiola Atzeni; Edoardo Rossi; Andrea Doria; Five-Year Follow-Up by Transesophageal Echocardiographic Studies in Primary Antiphospholipid Syndrome. The American Journal of Cardiology 2005, 96, 574-579, 10.1016/j.amjcard.2005.04.022.
- Fabiola Atzeni; Marco Corda; Luigi Gianturco; Maurizio Porcu; Piercarlo Sarzi-Puttini; Maurizio Turiel; Cardiovascular Imaging Techniques in Systemic Rheumatic Diseases. Frontiers in Medicine 2018, 5, 26, 10.3389/fmed.2018.00026.
- Carlo Palazzi; Carlo Salvarani; Salvatore D’Angelo; I. Olivieri; Aortitis and periaortitis in ankylosing spondylitis. Joint Bone Spine 2011, 78, 451-455, 10.1016/j.jbspin.2010.11.003.
- Charles R. Tucker; Robert E. Fowles; Andrei Calin; Richard L. Popp; Aortitis in ankylosing spondylitis: Early detection of aortic root abnormalities with two dimensional echocardiography. The American Journal of Cardiology 1982, 49, 680-686, 10.1016/0002-9149(82)91946-4.
- Hanna Przepiera-Bedzak; Iwona Brzosko; Małgorzata Peregud-Pogorzelska; Marek Wódecki; Marek Brzosko; [Cardiovascular manifestations of seronegative inflammatory spondyloarthropathies].. Annales Academiae Medicae Stetinensis 2010, 56, 62-65.
- Eva Klingberg; Bente Grüner Sveälv; Margareta Scharin Täng; Odd Bech-Hanssen; Lennart Bergfeldt; Helena Forsblad-D’Elia; Aortic Regurgitation Is Common in Ankylosing Spondylitis: Time for Routine Echocardiography Evaluation?. The American Journal of Medicine 2015, 128, 1244-1250.e1, 10.1016/j.amjmed.2015.04.032.
- Cecilia P. Chung; Annette Oeser; Paolo Raggi; Tebeb Gebretsadik; Ayumi K. Shintani; Tuulikki Sokka; Theodore Pincus; Ingrid Avalos; C. Michael Stein; Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Care & Research 2005, 52, 3045-3053, 10.1002/art.21288.
- Anna Abou-Raya; Suzan Abou-Raya; Inflammation: A pivotal link between autoimmune diseases and atherosclerosis. Autoimmunity Reviews 2006, 5, 331-337, 10.1016/j.autrev.2005.12.006.
- Martín-Martínez, Μ.A.; González-Juanatey, C.; Castañeda, S.; Llorca, J.; Ferraz-Amaro, I.; Fernández-Gutiérrez, B.; Díaz-González, F.; González-Gay, M.A. Recommendations for the management of cardiovascular risk in patients with rheumatoid arthritis: Scientific evidence and expert opinion. Semin. Arthritis Rheum. 2014, 44, 1–8.
- Ikonomidis, I.; Makavos, G.; Katsimbri, P.; Boumpas, D.T.; Parissis, J.; Iliodromitis, E. Imaging risk in multisystem inflammatory diseases. JACC Cardiovasc. Imaging 2019, 12, 2517–2537.
- Kerekes, G.; Soltész, P.; Nurmohamed, M.T.; Gonzalez-Gay, M.A.; Turiel, M.; Végh, E.; Shoenfeld, Y.; McInnes, I.; Szekanecz, Z. Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat. Rev. Rheumatol. 2012, 8, 224–234.
- Szekanecz, Z.; Kerekes, G.; Végh, E.; Kardos, Z.; Baráth, Z.; Tamási, L.; Shoenfeld, Y. Autoimmune atherosclerosis in 3D: How it develops, how to diagnose and what to do. Autoimmun. Rev. 2016, 15, 756–769.
- Alfonso Corrales; Carlos Gonzalez-Juanatey; María E Peiró; Ricardo Blanco; J. Llorca; Miguel Á. González-Gay; Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Annals of the Rheumatic Diseases 2013, 73, 722-727, 10.1136/annrheumdis-2012-203101.
- Gilles Montalescot; Udo Sechtem; Stephan Achenbach; Felicita Andreotti; Chris Arden; Andrzej Budaj; Raffaele Bugiardini; Filippo Crea; Thomas Cuisset; Carlo Di Mario; et al.J. Rafael FerreiraBernard J. GershAnselm K. GittJean-Sébastien HulotNikolaus MarxLionel H. OpieMatthias PfistererEva PrescottFrank RuschitzkaManel SabatéRoxy SeniorDavid Paul TaggartErnst E. Van Der WallChristiaan J.M. VrintsJose Luis ZamoranoHelmut BaumgartnerJeroen J. BaxHéctor BuenoVeronica DeanChristi DeatonÇetin ErolRobert FagardRoberto FerrariDavid HasdaiArno W. HoesPaulus KirchhofJuhani KnuutiPhilippe KolhPatrizio LancellottiAleš LinhartPetros NihoyannopoulosMassimo F. PiepoliPiotr PonikowskiPer Anton SirnesJuan TamargoMichal TenderaAdam TorbickiWilliam WijnsStephan WindeckerMarco ValgimigliMarc ClaeysNorbert Donner-BanzhoffHerbert FrankChristian Funck-BrentanoOliver GaemperliJosé R. Gonzalez-JuanateyMichalis HamilosSteen HustedStefan K. JamesKari KervinenSteen Dalby KristensenAldo Pietro MaggioniAxel R. PriesFrancesco RomeoLars RydénMaarten L. SimoonsPhilippe Gabriel StegAdam TimmisAylin Yildirir 2013 ESC guidelines on the management of stable coronary artery disease. European Heart Journal 2013, 34, 2949-3003, 10.1093/eurheartj/eht296.
- Mohammed K. Saghir; Christine Attenhofer Jost; Kenneth J. Warrington; Stephen S. Cha; Patricia A. Pellikka; Exercise Echocardiography in Rheumatoid Arthritis: A Case-Control Study. Journal of the American Society of Echocardiography 2009, 22, 1228-1231, 10.1016/j.echo.2009.07.018.
- Konstantinos Toutouzas; Petros P. Sfikakis; Antonios Karanasos; Constantina Aggeli; Ioannis Felekos; George D. Kitas; Evangelia Zampeli; Athanasios Protogerou; Christodoulos Stefanadis; Myocardial ischaemia without obstructive coronary artery disease in rheumatoid arthritis: hypothesis-generating insights from a cross-sectional study. Rheumatology 2012, 52, 76-80, 10.1093/rheumatology/kes349.
- Patrick Meimoun; Christophe Tribouilloy; Non-invasive assessment of coronary flow and coronary flow reserve by transthoracic Doppler echocardiography: a magic tool for the real world. European Journal of Echocardiography 2008, 9, 449-457, 10.1093/ejechocard/jen004.
- Lasse Jespersen; Anders Hvelplund; Steen Z. Abildstrøm; Frants Pedersen; Søren Galatius; Jan K. Madsen; Erik Jørgensen; H. Kelbaek; Eva Prescott; Henning Kelbæk; et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. European Heart Journal 2011, 33, 734-744, 10.1093/eurheartj/ehr331.
- Yang Ya; Thomas Bartel; Holger Eggebrecht; Loredana Latina; Clemems Von Birgelen; Guido Caspari; Wang Xinfang; Raimund Erbel; Non-invasive assessment of coronary flow velocity reserve: A new method using transthoracic Doppler echocardiography. Journal of Huazhong University of Science and Technology [Medical Sciences] 2002, 22, 158-163, 10.1007/bf02857683.
- Gian Luca Erre; Giorgio Buscetta; Panagiotis Paliogiannis; Arduino Aleksander Mangoni; Ciriaco Carru; Giuseppe Passiu; Angelo Zinellu; Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta-analysis. Rheumatology International 2018, 38, 1179-1190, 10.1007/s00296-018-4039-8.
- Maurizio Turiel; Fabiola Atzeni; Livio Tomasoni; Simona De Portu; Luigi Delfino; Bruno Dino Bodini; Matteo Longhi; Simona Sitia; Mauro Bianchi; Paolo Ferrario; et al.Andrea DoriaVito De Gennaro ColonnaPiercarlo Sarzi-Puttini Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology 2009, 48, 834-839, 10.1093/rheumatology/kep082.
- Sean G. O’Neill; Jose M. Pego-Reigosa; Aroon D. Hingorani; Rupa Bessant; David A. Isenberg; Anisur Rahman; Use of a strategy based on calculated risk scores in managing cardiovascular risk factors in a large British cohort of patients with systemic lupus erythematosus. Rheumatology 2008, 48, 573-575, 10.1093/rheumatology/kep037.
- Mary J. Roman; Beth-Ann Shanker; Adrienne Davis; Michael D. Lockshin; Lisa Sammaritano; Ronit Simantov; Mary K. Crow; Joseph E. Schwartz; Stephen A. Paget; Richard B. Devereux; et al.Jane E. Salmon Prevalence and Correlates of Accelerated Atherosclerosis in Systemic Lupus Erythematosus. New England Journal of Medicine 2003, 349, 2399-2406, 10.1056/nejmoa035471.
- Rupa Bessant; Rachel Duncan; Gareth Ambler; Jo Swanton; David A. Isenberg; Caroline Gordon; Anisur Rahman; Prevalence of conventional and lupus-specific risk factors for cardiovascular disease in patients with systemic lupus erythematosus: A case–control study. Arthritis Care & Research 2006, 55, 892-899, 10.1002/art.22343.
- Susan Manzi; Faith Selzer; Kim Sutton-Tyrrell; Shirley G. Fitzgerald; Joan E. Rairie; Russell P. Tracy; Lewis H. Kuller; Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Care & Research 1999, 42, 51-60, 10.1002/1529-0131(199901)42:1<51::aid-anr7>3.0.co;2-d.
- Kumiko Hirata; Amudha Kadirvelu; Mitsuyo Kinjo; Robert Sciacca; Kenichi Sugioka; Ryo Otsuka; Annamaria Choy; Sook K. Chow; Minoru Yoshiyama; Junichi Yoshikawa; et al.Shunichi HommaChim C. Lang Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis Care & Research 2007, 56, 1904-1909, 10.1002/art.22702.
- Ikonomidis, I.; Papadavid, E.; Makavos, G.; Andreadou, I.; Varoudi, M.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Moutsatsou, P.; et al. Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor-a antagonism or cyclosporine in psoriasis. Circ. Cardiovasc. Imaging 2017, 10, e006283.
- Makavos, G.; Ikonomidis, I.; Andreadou, I.; Varoudi, M.; Kapniari, I.; Loukeri, E.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Thymis, J.; et al. Effects of interleukin 17a inhibition on myocardial deformation and vascular function in psoriasis. Can. J. Cardiol. 2020, 36, 100–111.
- Szabo, S.M.; Levy, A.R.; Rao, S.R.; Kirbach, S.E.; Lacaille, D.; Cifaldi, M.; Maksymowych, W.P. Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: A population-based study. Arthritis Rheum. 2011, 63, 3294–3304.
- Ungprasert, P.; Srivali, N.; Kittanamongkolchai, W. Risk of coronary artery disease in patients with ankylosing spondylitis: A systematic review and meta-analysis. Ann. Transl. Med. 2015, 3, 51.
- Hafid Ait-Oufella; Andrew Sage; Ziad Mallat; Alain Tedgui; Adaptive (T and B Cells) Immunity and Control by Dendritic Cells in Atherosclerosis. Circulation Research 2014, 114, 1640-1660, 10.1161/circresaha.114.302761.
- Ait-Oufella, H.; Sage, A.P.; Mallat, Z.; Tedgui, A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ. Res. 2014, 114, 1640–1660.
- Mathieu, S.; Gossec, L.; Dougados, M.; Soubrier, M. Cardiovascular profile in ankylosing spondylitis: A systematic review and meta-analysis. Arthritis Care Res. 2011, 63, 557–563.
- McGettigan, P.; Henry, D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006, 296, 1633–1644.
- Nazzareno Galiè; Marc Humbert; J-L Vachiéry; Simon Gibbs; Irene M. Lang; Karol Adam Kaminski; Gérald Simonneau; Andrew Peacock; Anton Vonk Noordegraaf; Maurice Beghetti; et al.Hossein Ardeschir GhofraniMiguel Angel Gómez SánchezGeorg HansmannWalter KlepetkoPatrizio LancellottiMarco MatucciTheresa McDonaghLuc A. PierardPedro T. TrindadeMaurizio ZompatoriM.M. HoeperVictor AboyansAntonio Vaz CarneiroStephan AchenbachStefan AgewallYannick AllanoreMd Fesc Riccardo AsteggianoLuigi P. BadanoJoan Albert BarberàHélène BouvaistHéctor BuenoRobert A. ByrneScipione CarerjGraça CastroÇetin ErolVolkmar FalkChristian Funck-BrentanoMatthias GorenfloJohn GrantonBernard IungDavid G KielyPaulus KirchhofBarbro KjellstromUlf LandmesserJohn LekakisChristos LionisGregory Y H LipStylianos E OrfanosMyung H ParkMassimo F. PiepoliPiotr PonikowskiMarie-Pierre RevelDavid RigauStephan RosenkranzHeinz VöllerJose Luis ZamoranoLina BadimonGonzalo Barón-EsquiviasHelmut BaumgartnerJeroen J BaxVeronica DeanDonna FitzsimonsOliver GaemperliPhilippe KolhPetros NihoyannopoulosMarco RoffiStephan WindeckerSokol MyftiuDiana BondermanIbrahimov FirdovsiIrina LazarevaMichel De PauwŠekib SokolovicVasil VelchevMaja CikešJosef Antoniou MoutirisPavel JansaJens Erik Nielsen-KudskLy AntonPertti JääskeläinenFabrice BauerArchil ChukhrukidzeChristian OpitzGeorge GiannakoulasKristóf KarlócaiHjörtur OddssonSean GaineDoron MenachemiMichele EmdinTalant SooronbaevTalant Ainars RudzitisLina GumbieneFrederic LebrunJosef MicallefVictor BotnaruLatifa OukerrajArne K AndreassenMarcin KurzynaMaria João Ribeiro Leite BaptistaIoan Mircea ComanOlga MoiseevaBranislav S StefanovicIveta ŠimkováGerhard WikströmMarkus SchwerzmannElizabeta Srbinovska-KostovskaArie P. J. Van DijkAbdallah MahdhaouiCihangir KaymazJ. Gerry CoghlanYuriy Sirenko 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal 2015, 37, 67-119, 10.1093/eurheartj/ehv317.
- Kenya Kusunose; Hirortsugu Yamada; Junko Hotchi; Mika Bando; Susumu Nishio; Yukina Hirata; Takayuki Ise; Koji Yamaguchi; Syusuke Yagi; Takeshi Soeki; et al.Tetsuzo WakatsukiJun KishiMasataka Sata Prediction of Future Overt Pulmonary Hypertension by 6-Min Walk Stress Echocardiography in Patients With Connective Tissue Disease. Journal of the American College of Cardiology 2015, 66, 376-384, 10.1016/j.jacc.2015.05.032.
- Christian Cadeddu; Martino Deidda; Giuseppina Giau; Marzia Lilliu; Fabio Cadeddu; Giulio Binaghi; Mario Nicola Mura; Michela Farci; Stefano Del Giacco; Paolo Emilio Manconi; et al.Guiseppe Mercuro Contractile reserve in systemic sclerosis patients as a major predictor of global cardiac impairment and exercise tolerance. The International Journal of Cardiovascular Imaging 2014, 31, 529-536, 10.1007/s10554-014-0583-9.
- Roberto M. Lang; Luigi P. Badano; Victor Mor-Avi; Jonathan Afilalo; Anderson Da Costa Armstrong; Laura Ernande; Frank A. Flachskampf; Elyse Foster; Steven A. Goldstein; Tatiana Kuznetsova; et al.Patrizio LancellottiDenisa MuraruMichael H. PicardErnst R. RietzschelLawrence G. RudskiKirk T. SpencerWendy TsangJens-Uwe Voigt Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2015, 28, 1-39.e14, 10.1016/j.echo.2014.10.003.
- Yuichi J. Shimada; Maiko Shiota; Robert J. Siegel; Takahiro Shiota; Accuracy of Right Ventricular Volumes and Function Determined by Three-Dimensional Echocardiography in Comparison with Magnetic Resonance Imaging: A Meta-Analysis Study. Journal of the American Society of Echocardiography 2010, 23, 943-953, 10.1016/j.echo.2010.06.029.
