Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Caili Zhang.
Poly(butylene adipate-co-terephthalate) (PBAT) is a biodegradable polymer synthesized from petrochemical resources. PBAT has an exceptionally high elongation at break values which makes it one of the most promising substitutes for LDPE packaging films. However, the applicability of PBAT films is still limited by low strength and high production costs. In this wPork, we used polyethylene glycol 600 (PEG-600) was used as a coating agent to modify the surface of calcium carbonate and improve compatibility with the polymer matrix. A series of PBAT/CaCO3 composite films having different CaCO3 particle size and content of coating agent was prepared using extrusion blow molding.
- PBAT film
- PBAT
- Biodegradable films
1. Introduction
Poly(butylene adipate-co-terephthalate) (PBAT) is a biodegradable plastic with excellent mechanical properties. Although synthesized from petrochemical resources, it has been considered as the most viable substitute for LDPE [1,2,3,4,5,6,7,8][1][2][3][4][5][6][7][8]. Although PBAT appeared on the market about a decade ago, the use of neat PBAT film products is limited by the high production costs and lower strength compared with LDPE as the most used packaging film material [9,10,11][9][10][11].
The mechanical properties of PBAT films are often reinforced by the incorporation of low-cost fillers such as starch, CaCO3, and hydrotalcite into the polymer matrix [12,13,14,15,16,17][12][13][14][15][16][17]. Calcium carbonate is one of the most applied inorganic fillers in thermoplastics [18,19,20,21,22][18][19][20][21][22]. The properties of polymer/CaCO3 composites depend on the amount of filler added to the polymer matrix, and the application type determines the maximum loading of CaCO3. For example, mulching films require good light transmittance and therefore tolerate only small amounts of CaCO3 [23]. On the other hand, packaging films could be filled with up to 30% CaCO3 to reduce the price of polymer composite while maintaining satisfactory mechanical properties [24]. The particle size of CaCO3, its dispersibility in the polymer matrix, and the affinity of a polymer toward filler determine the overall mechanical properties of a composite [25,26,27][25][26][27]. However, the main issue with this type of composite is the agglomeration that originates from the incompatibility between the filler and a polymer.
The addition of coating agents greatly enhances the stability of composite materials [28]. There are two types of coating agents classified according to the type of intermolecular interactions: (1) dispersants, which interact with particle surface through non-covalent Van der Waals forces, and (2) coupling agents, which are attached to the particle surface via covalent bonds. Fatty acids are the most widely used dispersants for CaCO3 and were applied in LDPE/CaCO3, HDPE/CaCO3, and PP/CaCO3 composites [21,29,30][21][29][30]. Among coupling agents, silanes and titanates are often applied in the synthesis of composites [31].
2. Effect of CaCO3 Particle Size and Coating Agent Content on the Properties of PBAT/CaCO3 Films
2.1. Mechanical Properties
To better understand these trends, the variations in tensile strength and elongation at break have been showed in Figure 21. All properties of extruded films have been measured in the machine direction (MD) and the transverse direction (TD). The results revealed that the mechanical properties measured in both directions improve with the decrease of particle size from 12 μm to 5 μm. The superior performance of a sample P7C3-3 with the smallest particles is in accordance with the results of our previous work [32].
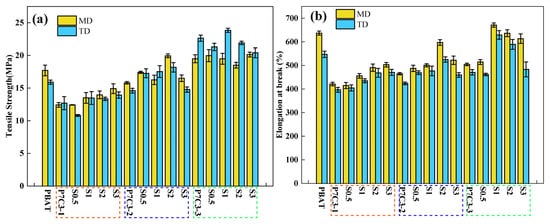

Figure 21.
(
a
) Tensile strength and (
b
) elongation at break of PBAT/CaCO
3
films.
Comparing two samples having the same mass, the one with a smaller particle size has a larger surface area, and it could be expected that more coating agent is required to effectively modify its surface. However, it was interesting to observe that the amount of coating agent needed for the material to reach the optimum mechanical performance decreased with the particle size reduction. For example, the optimal content of the coating agent was 3% in P7C3-1, 2% in P7C3-2, and 1% in the P7C3-3 sample. This effect can be explained by strong electrostatic interactions between smaller CaCO3 particles that impede the dispersion and surface modification of individual particles.
2.2. Rheological Properties
Rheological properties of molten composites strongly depend on the dispersibility of filler particles within the polymer matrix and the nature of intermolecular interactions. The effect of the coating agent on the structure and dispersion state of CaCO3 within the polymer matrix has been studied by measuring the complex viscosity and storage modulus of several composites modified by different amounts of PEG. These parameters have been compared with the values for neat PBAT (Figure 32).
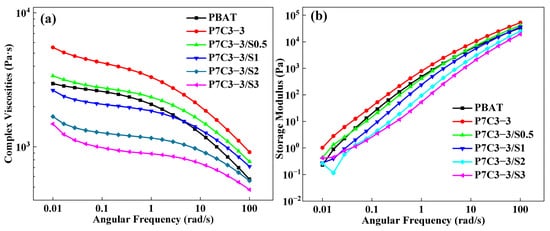

Figure 32. (a) Complex viscosity and (b) storage modulus of PBAT/CaCO3 films prepared using calcium carbonate with particle size 300 mesh and different amounts of PEG coating agent.
2.3. Surface Morphology
The changes in the surface microstructure of PBAT films upon the addition of CaCO3 filler and PEG coating agent have been studied using SEM. The surface of unmodified PBAT/CaCO3 films appeared relatively rough with abundant particle aggregates. On the other hand, composite films modified by the PEG coating agent exhibited relatively flat and smooth surface with good particle dispersion and without pronounced agglomeration. This confirms that the coating agent effectively increases the compatibility between PBAT and fillers.
3. Degradation Behavior of PBAT/CaCO3 Composites in Soil and Simulated Seawater Environment
The biodegradation of plastic films in soil or seawater is a complex process governed by the interplay of biological and physicochemical degradation mechanisms. The degradation processes that occur in seawater are somewhat different from those in the soil as seawater is rich in inorganic salts, deficient in organic nutrients, and has a weakly alkaline pH. In addition, the concentration of dissolved O2 is much lower in seawater compared with the soil environment which also influences the biodegradation rate of plastics [40][33]. In tThis study, we examined the biodegradation of PBAT/CaCO3 composites in soil and seawater was examed.
3.1. Visual Inspection of Structural Changes upon Biodegradation of PBAT/CaCO3 Composites in Soil and Simulated Seawater Environment
The initial thickness of the composite film was 0.055 ± 0.015 mm. After 30 days in the soil environment, we observed partial fragmentation and erosion holes, which was accompanied by the decrease of film thickness to 0.046 ± 0.018 mm. After another 30 days of environmental exposure, the composite films were essentially broken into fragments and the average film thickness was reduced to 0.029 ± 0.013 mm. The P7C3-2/S0.5, P7C3-2/S1, and P7C3-3 samples were almost completely degraded which complicated the sampling for the analysis. After 90 days in soil, all samples except P7C3-1, P7C3-3/S2, and P7C3-3/S3 were almost completely decomposed and the film thickness was reduced to 0.012 ± 0.008 mm.
The biodegradation processes in simulated seawater environment were much slower compared to soil. It took 60 days to observe the first changes in film thickness, and it was reduced from the initial value to 0.050 ± 0.012 mm. After 90 days in seawater, PBAT/CaCO3 composites changed the color to brown and some signs of fragmentation occurred. Moreover, the film thickness dropped to 0.044 ± 0.016 mm. The images of neat PBAT, P7C3-3, and P7C3-3/S1 films taken before and after biodegradation in soil and seawater are given in Figure 43.
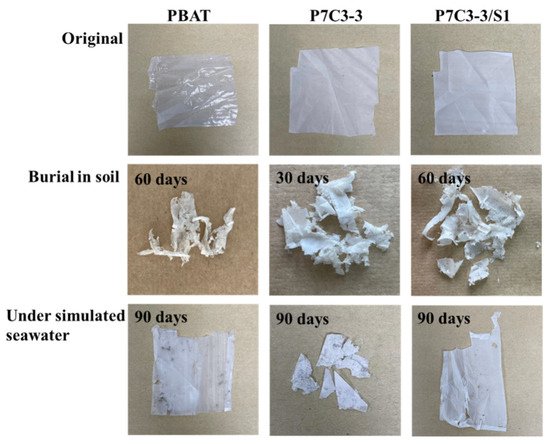

Figure 43. The optical images of neat PBAT, P7C3-3, and P7C3-3/S1 films before and after biodegradation in soil and simulated seawater.
3.2. The Changes in Molecular Weight of Polymer upon Biodegradation Treatments
The molecular weight (MW) of the polymer was measured to further characterize the decomposition of neat PBAT films and PBAT/CaCO3 composites after two biodegradation treatments mentioned before.
For all samples, Mn steadily decreased with soil degradation time. Comparing the degree of degradation for neat PBAT and PBAT composites with CaCO3 having various particle size, it decreased in the following order: P7C3-3 > P7C3-2 ≈ PBAT > P7C3-1. Two samples with the smallest particle size, namely P7C3-2 and P7C3-3 were almost completely decomposed after 90 and 60 days, respectively. This trend might be explained by the larger surface area of composite films filled with smaller CaCO3 particles, providing more opportunities for contact with water and oxygen molecules, thus facilitating the diffusion of water and hydrolysis/biodegradation of PBAT.
This trend could be related to the previous discussion on the mechanical properties of PBAT/CaCO3 composites. When the amount of coating agent exceeds optimum, the dispersion of coated reinforcements being less successful, and the diffusion of water is less favored, this effect reduces the hydrolysis/biodegradation of the PBAT matrix. These results highlight the importance of the optimization of coating agent loading for mechanical and biodegradation film performances.
The molecular weight measurements also confirmed that the degradation rate in seawater is much slower than in soil. Although particle size and coating agent influence the diffusivity of water in the polymer matrix, it was found that the degradation rate of P7C3-3 and P7C3-3/S0.5 was similar to that of neat PBAT film. These results demonstrate the dominant role of biodegradation in the overall decomposition mechanism of PBAT/CaCO3 composites while hydrolysis has a minor influence. Some studies also revealed that the type and size of the microbial community are the key parameters for the biodegradation rate of plastics [42][34].
3.3. FTIR Analysis of Changes in the Chemical Structure of PBAT upon Biodegradation
The changes in the structure of PBAT polyester caused by soil or seawater degradation have been studied by FTIR. The FTIR spectra of pure PBAT and different PBAT/CaCO3 composite films are shown in Figure 64. Two intense peaks observed at 1710 cm−1 and 1250 cm−1 originate from the stretching vibrations of carbonyl (C=O) and aliphatic C-O-C bonds of ester groups [43][35]. Soil degradation causes a significant decrease in the intensity of two ester peaks after 30 days of treatment, and the effect intensifies after the additional 30 days. Seawater biodegradation also reduces the intensity of FTIR ester peaks of PBAT but to a much lower extent. Moreover, the changes in FTIR spectra are observed in the fingerprint region. It can be concluded that the biodegradation of PBAT polyester proceeds mainly through the hydrolysis of ester bonds.
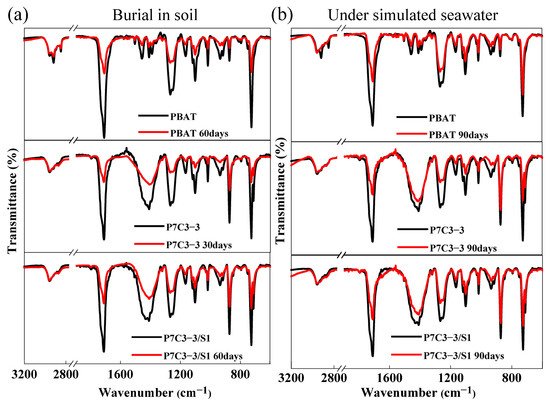

Figure 64. FTIR spectra of neat PBAT, P7C3-3, and P7C3-3/S1 films after 30, 60, or 90 days of biodegradation in (a) soil and (b) simulated seawater environment.
3.4. The Changes in the Morphology of Polymers Caused by Biodegradation Revealed by SEM
The microstructure of neat PBAT, P7C3-3 composite, and composite with PEG-coated filler have been observed before and after each biodegradation test. According to SEM micrographs shown in Figure 75, the smooth surface of PBAT was preserved after 60 days of degradation in soil and only small pores and cracks occurred. Somewhat rougher surface of P7C3-3 films transformed into the structure with large pores and small cracks after 30 days in the soil environment. In case of surface-coated P7C3-3/S1 films, large cracks appeared at the surface after 60 days in soil.


Figure 75. SEM images of (a) neat PBAT, (b) P7C3-3 and (c) P7C3-3/S1 films before and after biodegradation treatments. (a’–c’): burial in soil degradation; (a”–c”): under simulated seawater degradation.
The biodegradation in a simulated seawater environment resulted in significantly lesser changes in the microstructure of PBAT and composites. After 90 days of treatment, no visible signs of biodegradation have been observed in neat PBAT film. More pronounced structure defects appeared in P7C3-3 and P7C3-3/S1 films after 90 days in simulated seawater. The swelling of these polymers due to diffusion of water into the matrix resulted in the departure of CaCO3 particles and the occurrence of holes that could be observed from SEM images. In addition, no visible cracks were found for these samples. These results additionally support previous findings that CaCO3 as a filler and hydrophilic PEG as a coating agent promote the diffusion of water in a polymer matrix.
References
- Zhang, T.; Han, W.; Zhang, C.; Weng, Y. Effect of chain extender and light stabilizer on the weathering resistance of PBAT/PLA blend films prepared by extrusion blowing. Polym. Degrad. Stabil. 2021, 183, 109455.
- Khan, H.; Kaur, S.; Baldwin, T.C.; Radecka, I.; Jiang, G.; Bretz, I.; Duale, K.; Adamus, G.; Kowalczuk, M. Effective Control against Broadleaf Weed Species Provided by Biodegradable PBAT/PLA Mulch Film Embedded with the Herbicide 2-Methyl-4-Chlorophenoxyacetic Acid (MCPA). ACS Sustain. Chem. Eng. 2020, 8, 5360–5370.
- Jiang, G.; Li, H.; Wang, F. Structure of PBAT/PPC blends prepared by in-situ reactive compatibilization and properties of their blowing films. Mater. Today Commun. 2021, 27, 102215.
- Chang, C.C.; Trinh, B.M.; Mekonnen, T.H. Robust multiphase and multilayer starch/polymer (TPS/PBAT) film with simultaneous oxygen/moisture barrier properties. J. Colloid. Interface Sci. 2021, 593, 290–303.
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26.
- Camani, P.H.; Souza, A.G.; Barbosa, R.F.S.; Zanini, N.C.; Mulinari, D.R.; Rosa, D.S. Comprehensive insight into surfactant modified-PBAT physico-chemical and biodegradability properties. Chemosphere 2021, 269, 128708.
- Shar, A.S.; Zhang, C.; Song, X.; Weng, Y.; Du, Q. Design of novel PLA/OMMT films with improved gas barrier and mechanical properties by intercalating OMMT interlayer with high gas barrier polymers. Polymers 2021, 13, 3962.
- Li, Y.; Zhao, L.; Changyu, H.; Yancun, Y. Biodegradable blends of poly (butylene adipate-co-terephthalate) and stereocomplex polylactide with enhanced rheological, mechanical properties and thermal resistance. Colloid Polym. Sci. 2020, 298, 463–475.
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr. Polym. 2020, 239, 116231.
- Bai, J.; Pei, H.; Zhou, X.; Xie, X. Reactive compatibilization and properties of low-cost and high-performance PBAT/thermoplastic starch blends. Eur. Polym. J. 2021, 143, 110198.
- Wei, X.; Ren, L.; Sun, Y.; Zhang, X.; Guan, X.; Zhang, M.; Zhang, H. Sustainable composites from biodegradable poly (butylene succinate) modified with thermoplastic starch and poly (butylene adipate-co-terephthalate): Preparation and performance. New J. Chem. 2021, 45, 17384–17397.
- Xie, J.; Wang, Z.; Zhao, Q.; Yang, Y.; Xu, J.; Waterhouse, G.I.N.; Zhang, K.; Li, S.; Jin, P.; Jin, G. Scale-Up Fabrication of Biodegradable Poly(butylene adipate-co-terephthalate)/Organophilic-Clay Nanocomposite Films for Potential Packaging Applications. ACS Omega 2018, 3, 1187–1196.
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.-J. Biodegradable UV-Blocking Films through Core–Shell Lignin–Melanin Nanoparticles in Poly(butylene adipate-co-terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157.
- Rocha, D.B.; Souza de Carvalho, J.; de Oliveira, S.A.; dos Santos Rosa, D. A new approach for flexible PBAT/PLA/CaCO3 films into agriculture. J. Appl. Polym. Sci. 2018, 135, 46660.
- Li, J.; Lai, L.; Wu, L.; Severtson, S.J.; Wang, W.-J. Enhancement of Water Vapor Barrier Properties of Biodegradable Poly(butylene adipate-co-terephthalate) Films with Highly Oriented Organomontmorillonite. ACS Sustain. Chem. Eng. 2018, 6, 6654–6662.
- Girdthep, S.; Worajittiphon, P.; Leejarkpai, T.; Molloy, R.; Punyodom, W. Effect of silver-loaded kaolinite on real ageing, hydrolytic degradation, and biodegradation of composite blown films based on poly(lactic acid) and poly(butylene adipate-co-terephthalate). Eur. Polym. J. 2016, 82, 244–259.
- Mondal, D.; Bhowmick, B.; Mollick, M.M.R.; Maity, D.; Ranjan Saha, N.; Rangarajan, V.; Rana, D.; Sen, R.; Chattopadhyay, D. Antimicrobial activity and biodegradation behavior of poly(butylene adipate-co-terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2014, 131, 400079.
- Zuiderduin, W.C.J.; Westzaan, C.; Huétink, J.; Gaymans, R.J. Toughening of polypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275.
- Xie, X.-L.; Liu, Q.-X.; Li, R.K.-Y.; Zhou, X.-P.; Zhang, Q.-X.; Yu, Z.-Z.; Mai, Y.-W. Rheological and mechanical properties of PVC/CaCO3 nanocomposites prepared by in situ polymerization. Polymer 2004, 45, 6665–6673.
- Zapata, P.A.; Palza, H.; Diaz, B.; Armijo, A.; Sepulveda, F.; Ortiz, J.A.; Ramirez, M.P.; Oyarzun, C. Effect of CaCO(3) Nanoparticles on the Mechanical and Photo-Degradation Properties of LDPE. Molecules 2018, 24, 10126.
- Mantia, F.P.L.; Morreale, M.; Scaffaro, R.; Tulone, S. Rheological and mechanical behavior of LDPE/calcium carbonate nanocomposites and microcomposites. J. Appl. Polym. Sci. 2013, 127, 2544–2552.
- Piekarska, K.; Piorkowska, E.; Bojda, J. The influence of matrix crystallinity, filler grain size and modification on properties of PLA/calcium carbonate composites. Polym. Test 2017, 62, 203–209.
- Titone, V.; La Mantia, F.P.; Mistretta, M.C. The Effect of Calcium Carbonate on the Photo-Oxidative Behavior of Poly(butylene adipate-co-terephthalate). Macromol. Mater. Eng. 2020, 305, 2000358.
- Luo, Z.; Wang, Y.; Jiang, L.; Xu, X. Effect of nano-CaCO3-LDPE packaging on quality and browning of fresh-cut yam. LWT Food Sci. Technol. 2015, 60, 1155–1161.
- Sun, S.; Li, C.; Zhang, L.; Du, H.L.; Burnell-Gray, J.S. Interfacial structures and mechanical properties of PVC composites reinforced by CaCO3 with different particle sizes and surface treatments. Polym. Int. 2006, 55, 158–164.
- Oladele, I.O.; Ibrahim, I.O.; Akinwekomi, A.D.; Talabi, S.I. Effect of mercerization on the mechanical and thermal response of hybrid bagasse fiber/CaCO3 reinforced polypropylene composites. Polym. Test 2019, 76, 192–198.
- Zhao, H.; Yu, Y.; Han, C.; Liu, Q.; Liu, H.; Zhou, G.; Xu, M. Improving the stereocomplexation and toughness of poly (L-lactic acid)/poly (D-lactic acid) blends via melt blending with ethylene/methyl acrylate/glycidyl methacrylate terpolymer. J. Macromol. Sci. Part A 2021, 58, 419–430.
- Rong, M.; Zhang, M.; Ruan, W. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol-Lond. 2006, 22, 787–796.
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329.
- Sahebian, S.; Zebarjad, S.M.; Sajjadi, S.A.; Sherafat, Z.; Lazzeri, A. Effect of both uncoated and coated calcium carbonate on fracture toughness of HDPE/CaCO3 nanocomposites. J. Appl. Polym. Sci. 2007, 104, 3688–3694.
- Doufnoune, R.; Chebira, F.; Haddaoui, N. Effect of titanate coupling agent on the mechanical properties of calcium carbonate filled polypropylene. Int. J. Polym. Mater. Polym. Biomater. 2003, 52, 967–984.
- Zhang, T.; Zhang, C.; Yang, Y.; Yang, F.; Zhao, M.; Weng, Y. Improved properties of poly(butylene adipate-co-terephthalate)/calcium carbonate films through silane modification. J. Appl. Polym. Sci. 2021, 138, 50970.
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189.
- Wang, G.X.; Huang, D.; Ji, J.H.; Volker, C.; Wurm, F.R. Seawater-Degradable Polymers-Fighting the Marine Plastic Pollution. Adv. Sci. (Weinh) 2020, 8, 2001121.
- Qi, R.; Jones, D.L.; Liu, Q.; Liu, Q.; Li, Z.; Yan, C. Field test on the biodegradation of poly (butylene adipate-co-terephthalate) based mulch films in soil. Polym. Test 2021, 93, 107009.
More
