Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Cristina Andreea Adam.
Cardiac rehabilitation (CR) is an integral part of the management of various cardiovascular disease such as coronary artery disease (CAD), peripheral artery disease (PAD), or chronic heart failure (CHF), with proven morbidity and mortality benefits. Supervised exercise therapy has a positive impact on both functional capacity and also on the quality of life of such patients. The most effective manner to acquire this seems to be by combining revascularization therapy and supervised exercise.
- cardiac rehabilitation
- peripheral artery disease
- intermittent claudication
1. Treadmill Exercise
CR programs based on either supervised treadmill exercise or home-based walking exercise improve walking ability in PAD patients. A total of 3 randomized trials in which a number of 493 patients diagnosed with PAD were included, demonstrated that home-based walking exercise programs combined with behavioral change techniques improves the 6-min walk test performance more than supervised treadmill exercise interventions (45–54 m vs. 33–35 m) as well as walking ability [1][2].
In 1995, Gardner et al. performed a meta-analysis of 21 studies on PAD patients and concluded that supervised treadmill walking improved maximum treadmill walking distance from 125.9 ± 57.3 m to 351.2 ± 188.7 m (p < 0.001, increase by 179%) and pain-free treadmill walking distance from 325.8 ± 148.1 m to 723.3 ± 591.5 m (p < 0.001, increase by 122%) [3]. They also identified that increases in the distances to onset and to maximal claudication pain during treadmill exercise are independently related to three essential parts of a CR program which can be considered predictors of the changes in claudication pain distances: claudication pain end point used during the exercise training program, the length of the program and the type of exercise. Based on the results from the meta-regression analysis, Gardner et al. concluded that the most effective exercise programs for patients with PAD include 3 sessions per week, 30 min each, at intensity close to the point of maximum or near-maximum pain onset during exercise for at least 6 months [2][4].
Later, in 2012, Fakhry et al. summarized in a meta-analysis the results of 25 randomized clinical trials of supervised walking CR programs in which 1054 symptomatic PAD patients were included. Improvements in both maximal walking distance and pain-free walking distance were achieved in supervised walking exercise group (increase of 180 m, 95% CI, 130–230 m and 128 m, 95% CI, 92–165 m), compared to the control group without exercise. A total of 60% of the trials had a total duration between 12 and 26 weeks. In a subgroup analysis based on the length of the programs (<12 weeks, 12–26 weeks and >26 weeks), using multivariate meta-regression, Fakhry et al. observed the tendency to greater mean improvement in maximum walking distance and pain free walking distance in programs with a duration of 12–26 weeks, that those shorter or longer duration, suggesting a maximum benefit for PAD patients enrolled in CR programs with a duration between 12 and 26 weeks, with 3 sessions per week and 30 min of walking in each session [3][5]. The meta-analysis demonstrated significant functional benefits in treadmill walking performance in patients with PAD after finalizing the supervised CR program, with the reported results suggesting a lower final effect than the one obtained in the meta-analysis reported by Gardner et al. since it included only randomized trials [6][2].
1.1. Intensity
It is unclear if walking up to the maximal ischemic pain or rather just up to pain’s onset is more beneficial for the PAD patients, moreover since available trials did not show any difference between these strategies [7].
1.2. Program Length
Fakhry et al. reported significant increases in both pain-free treadmill walking time and maximum treadmill walking time regardless of CR program length (short: 4–11 weeks, medium: 12–26 weeks and long: more than 26 weeks). After 4 weeks of exercise the initial benefit is observed while the maximum benefit of the treadmill walking is achieved after 8–12 weeks of CR. The parameters associated with the 6-min walk test gradually improve due to the fact that the treadmill exercise trains the patient to measure the treadmill walking result (Figure 1) [2].
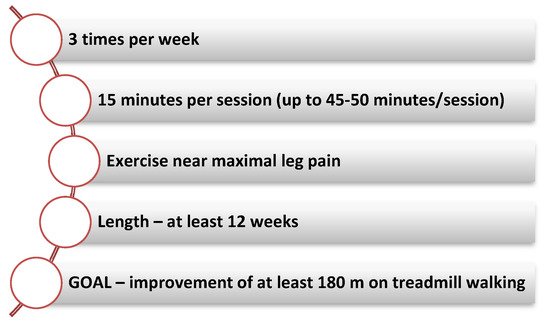
Figure 1.
Key elements of an exercise training program.
2. Home-Based Walking Exercise
Home-based exercise, including behavioral changes, represents an acceptable and affordable alternative to supervised weekly exercise, as it saves time and effort associated to traveling to a dedicated medical center. Home-based walking exercises are more accessible and easier to accept for PAD patients. Regardless of the presence or absence of symptoms, they improve both treadmill walking performance and walk distance in the 6MWT [2][4][8][9]. Furthermore, the benefits of home-based walking sessions in improving both walking capacity and the 6MWT parameters, compared to supervised treadmill exercise programs, have been proved since 2011 through several randomized trials.
Gardner et al. enrolled 119 men and women with symptomatic PAD to 1 of 3 groups (supervised treadmill exercise, home-based walking exercise, or a control group) for a total duration of 12 weeks. Patients randomly assigned to the home exercise group were instructed to perform exercise or walking sessions of at least 45 min, 3 times a week, at their own pace. At the 12-week follow-up, both patients from the home exercise group and the supervised exercise group showed a remarkable improvement in walking distance without the occurrence of IC and an increasing of the maximum exercise duration compared to the control group. Adherence to exercise programs was similar in the 2 groups (p > 0.05). PAD patients from the first group walked longer in each session (p < 0.001), but with a slower cadence than those in the second group (p < 0.05), resulting in a similar total exercise volume, expressed as MET-minutes (p > 0.05). No statistically significant differences were identified between the two groups in terms of treadmill walking ability or perimeter walking without IC. It is noteworthy that the study had an overall dropout rate of 23% in the home exercise group and 28% in the supervised treadmill exercise group, pointing difficulties in terms of adherence for PAD patients especially during COVID-19 pandemic [2][4][8][9][10][11].
In the second randomized trial, Gardner et al. randomized 180 PAD patients with IC to 3 groups: supervised treadmill exercise, home-based walking exercise and a light resistance training group. At the 12-week follow-up, patients from the first group had significantly greater improvement in treadmill walking compared to home-based exercise (192 ± 190 s vs. 110 ± 193 s vs. 22 ± 159 s) and in the time to onset of claudication pain on the treadmill (+170 s vs. +104 s vs. +17 s). Beneficial effects were also observed in the 6-minute walking distance which improved by 45 m in the home-based walking group compared to 15 m in the supervised treadmill group and 4 m in the control one [5][10].
The Group Oriented Arterial Leg Study (GOALS) is the only randomized clinical trial of home-based exercise for PAD patients both with and without IC. A total of 192 participants were randomized to a Group Mediated Cognitive Behavioral (GMCB) intervention group or to a control group. The GMCB intervention methods included social cognitive behavioral change theory and group support in order to increase adherence to home-based walking exercise programs and therefore increasing the walking performance. The intervention group had weekly meetings at the medical center with other PAD patients and a facilitator. At the 6 months follow-up, the intervention group had a significantly improved 6-minute walk performance compared to the control group (+42.4 m vs. 11.1 m). Improvements were also observed in the case of pain-free treadmill walking time (+1.01 min compared to the control group) and in maximum treadmill walking time. Support sessions were discontinued after the first 6 months, but the benefits on functional status persisted at the 12-month follow-up [9][12][13].
CR programs for PAD patients should be permanently adapted to the associated comorbidities and needs in order to achieve the desired results. The aim is to achieve a total exercise session duration of up to 50 min, with a gradual increase of 5 min each week, starting from a minimum duration of 30 min per session. The PAD patient should walk until close to reaching maximum leg pain. Even so, trials demonstrated that walking until the onset of intermittent claudication is also beneficial. Rest breaks are acceptable for PAD patients, with the recommendation to resume walking exercise as soon as leg pain has subsided [2][4][8][9][10][11].
Collins et al. also investigated the role of behavioral intervention methods on the adherence of PAD patients to home-based exercise programs. A total of 145 patients with PAD and diabetes were enrolled for 6 months and randomized into 2 groups: a behavioral intervention group vs. an attention control group. The patients from the first group had an individualized counseling session at enrollment, followed by a walking session weekly with an instructor and other patients with PAD at an exercise center and 3 days of walking at home (with a total of 50 min of exercise per session). All patients received bi-weekly phone calls, to evaluate progress in the first group. Compared to the previous study, at the six-month follow-up the investigators found no statistically significant differences in treadmill walking parameters between the two groups [14][9][15].
The impact of COVID-19 pandemic on home-based CR programs has been assessed by Lamberti et al. in a study in which 83 patients with PAD were enrolled within 9-month before the lockdown. The physical activity consisted of twice a day 8 min sessions of slow and intermittent in-home walking. During lockdown, the patients received regular telephone questionnaires regarding general health, adherence to exercise program and evolution of symptoms. Only 80% of the PAD patients showed up for the follow-up after lockdown. The pain-free walking distance improved improved directly proportional to the time since enlistment before the lockdown (p < 0.001) regardless of gender and comorbidities. Improvements were also observed regarding body weight, blood pressure and ankle-brachial index [16].
3. Alternative Forms of Exercise–Ergometry, Cycling and Strength Training
3.1. Ergometry
Classic CR programs such as home-based walking exercise or supervised treadmill exercise have more entertaining or challenging alternatives such as cycling, strength training, and upper arm ergometry. Upper-limb exercise determine greater heart rate, intra-arterial blood pressure, and pulmonary ventilation than lower-limb exercise for a specific level of submaximal work [17][18][19]. Multiple randomized trials concluded that both upper- and lower-extremity ergometry have significantly functional benefits especially improving walking endurance in PAD [20].
Zwierska et al. randomized 104 PAD patients into 3 major groups: upper-limb aerobic ergometry, lower-limb aerobic ergometry and a non-exercise control group for a total duration of CR program of 6 months. Each patient performed 2 weekly exercise sessions consisting of 10 cycles of 2 min each of arm (or leg) ergometer cycling, followed by 2 min of rest, with a total duration of 20 min of exercise each session. At the 6-month follow-up, maximum walking distance increased in both upper-limb and lower-limb ergometry groups (29% in the first group and 31% the second group). A beneficial effect was also identified in the peak oxygen uptake, suggesting a link between walking endurance and cardiovascular fitness [11].
Another alternative exercise modality for patients with PAD and IC is arm cranking. Tew et al. randomized a total of 57 patients with IC to an arm-crank exercise group and a non-exercise control group. Patients were evaluated at baseline and at the 12 weeks follow-up. The results showed an incremental improvement to maximum exercise tolerance on both an arm-crank ergometer and a treadmill. In the study group, increases in walking distance (from 496 ± 250 to 661 ± 324 m) and VO2 maximal values (from 17.2 ± 2.7 to 18.2 ± 3.4 mL·kg−1 body mass·min−1) were recorded in the treadmill walking test (p < 0.05). After training, an increase in time to reach minimum tissue O2 saturation (from 268 ± 305 s to 410 ± 366 s), as well as an increase in VO2 kinetics (from 44.7 ± 10.4 to 41.3 ± 14.4 s) and an increase in submaximal StO2 were observed during the treadmill walking test (p < 0.05). The increase in walking distance without the occurrence of IC as well as in maximum walking perimeter after arm crank exercise in patients with PAD is partly attributed to improved lower-limb oxygen delivery [21][22][23].
Walker et al. enrolled 67 patients with moderate and severe IC and randomized them in an upper-limb training group (26 patients), a lower-limb training group (26 patients) and a control group (15 patients). The patients from the training group had twice a week 40-min exercise sessions (2 min of exercise followed by 2 min of rest), for 6 weeks. At the follow-up, the pain free walking distance increased by 122% in the first group and by 93% in the second one (p < 0.001). Improvements have also been observed in the maximum walking distance which increased by 47% in the upper-limb group (p < 0.05) and by 50% in the lower-limb group (p < 0.001) [20][24].
3.2. Cycling
Lauret et al. included 135 patients from 5 studies that compared different training modalities, including supervised walking exercises. From the results, there was no statistically significant difference regarding reaching the maximum walking distance between supervised walking group and alternative training modalities (8.15 metabolic equivalent <METs>, 95% CI −2.63 to 18.94, p > 0.05, equivalent to an increase of 173 m, 95% CI −56 m to 401 m), on the treadmill without incline, for an average speed comparable to everyday life walking (3.2 km/h). At the same time, no statistically significant differences were observed for reaching the maximum walking perimeter without the occurrence of intermittent claudication (6.42 METs, 95% CI −1.52 to 14.36, p > 0.05, equivalent to an increase of 136 m, 95% CI −32 m to 304 m), with parameters associated with quality-of-life showing important improvements in both groups [23][25].
Sanderson et al. randomized 42 patients with PAD and IC in 3 different groups: a treadmill exercise group, a cycling one and a control group. The first 2 groups trained 3 times a week for 6 weeks. The exercise consisted of 10 rounds of 2 min each of exercise, interspersed with 2-min breaks, for a total of 20 min of exercise in each session. Cycle training improved cycle performance, but not walking performance. Treadmill training improved maximal and pain-free exercise time by 25%, but not maximal cycle time. While IC in the calf was the most common symptom in the first group, patients from the second group noted the presence of IC in the quadriceps. The difference between the location of IC in the two modes of exercise raise the observation that cycling would not have functional benefit for PAD patients who frequently experience IC in the calf. Sanderson et al. observed in PAD patients with limiting symptoms during cycling and walking a cross-transfer effect between the training modes, suggesting cycling as an exercise alternative for these patients [20][26].
3.3. Resistance Exercise Training
Strength training has been used in randomized trials to demonstrate the potential role in improving walking performance for PAD patients. Studies have shown that lower extremity strength training improves maximal treadmill walking time compared to a non-exercise control group. Following resistance training there is an increase in lower extremity skeletal muscle capillary growth. McDermott et al. reported no change in the primary outcome of 6-min walk distance in the strength training group, while supervised treadmill exercise significantly improved 6-min walk distance, leading to the conclusion that walking exercise is more effective than strength training in PAD patients [2][20].
Gomes et al. conducted a randomized controlled trial to evaluate resistance training effects on cardiovascular function. In total, 30 patients with PAD were enrolled and randomly allocated to a control group (15 patients, stretching and relaxation exercises) or resistance training group (15 patients, 3 sets of 10 repetitions of eight whole body exercises, with a pause of 2 minutes between sets). Resting and 24-h blood pressure (BP), cardiac output, systemic vascular resistance, and autonomic variables were obtained before and after 12 weeks of intervention. There was a time effect reduction in heart rate as well as statistically significant changes in cardiac autonomic modulation (p < 0.05). In the resistance training group, the blood pressure variability decreased in systolic, diastolic, and mean values (p < 0.05). At the 12-week follow-up the resting and 24-h BP, or their hemodynamic and autonomic determinants did not change in the PAD patients enrolled in the training group. However, there were decreases in BP variability, indicating that it could be considered as an alternative to reduce cardiovascular risk [27][2][20][28][29][30].
References
- Tran, B. Assessment and Management of Peripheral Arterial Disease: What Every Cardiologist Should Know. Heart Br. Card. Soc. 2021, 107, 1835–1843.
- McDermott, M.M.; Ades, P.; Guralnik, J.M.; Dyer, A.; Ferrucci, L.; Liu, K.; Nelson, M.; Lloyd-Jones, D.; Van Horn, L.; Garside, D.; et al. Treadmill Exercise and Resistance Training in Patients with Peripheral Arterial Disease with and without Intermittent Claudication: A Randomized Controlled Trial. JAMA 2009, 301, 165–174.
- Gardner, A.W.; Poehlman, E.T. Exercise Rehabilitation Programs for the Treatment of Claudication Pain. A Meta-Analysis. JAMA 1995, 274, 975–980.
- Sami, F.; Ranka, S.; Lippmann, M.; Weiford, B.; Hance, K.; Whitman, B.; Wright, L.; Donaldson, S.; Boyer, B.; Gupta, K. Cardiac Rehabilitation in Patients with Peripheral Arterial Disease after Revascularization. Vascular 2021, 29, 350–354.
- McDermott, M.M. Exercise Training for Intermittent Claudication. J. Vasc. Surg. 2017, 66, 1612–1620.
- Fakhry, F.; van de Luijtgaarden, K.M.; Bax, L.; den Hoed, P.T.; Hunink, M.G.M.; Rouwet, E.V.; Spronk, S. Supervised Walking Therapy in Patients with Intermittent Claudication. J. Vasc. Surg. 2012, 56, 1132–1142.
- Thomas, S.G.; Marzolini, S.; Lin, E.; Nguyen, C.H.; Oh, P. Peripheral Arterial Disease: Supervised Exercise Therapy Through Cardiac Rehabilitation. Clin. Geriatr. Med. 2019, 35, 527–537.
- Li, Y.; Li, Z.; Chang, G.; Wang, M.; Wu, R.; Wang, S.; Yao, C. Effect of Structured Home-Based Exercise on Walking Ability in Patients with Peripheral Arterial Disease: A Meta-Analysis. Ann. Vasc. Surg. 2015, 29, 597–606.
- McDermott, M.M.; Liu, K.; Guralnik, J.M.; Criqui, M.H.; Spring, B.; Tian, L.; Domanchuk, K.; Ferrucci, L.; Lloyd-Jones, D.; Kibbe, M.; et al. Home-Based Walking Exercise Intervention in Peripheral Artery Disease: A Randomized Clinical Trial. JAMA 2013, 310, 57–65.
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M. Step-Monitored Home Exercise Improves Ambulation, Vascular Function, and Inflammation in Symptomatic Patients With Peripheral Artery Disease: A Randomized Controlled Trial. J. Am. Heart Assoc. 2014, 3, e001107.
- Zwierska, I.; Walker, R.D.; Choksy, S.A.; Male, J.S.; Pockley, A.G.; Saxton, J.M. Upper- vs Lower-Limb Aerobic Exercise Rehabilitation in Patients with Symptomatic Peripheral Arterial Disease: A Randomized Controlled Trial. J. Vasc. Surg. 2005, 42, 1122–1130.
- McDermott, M.M.; Guralnik, J.M.; Criqui, M.H.; Ferrucci, L.; Liu, K.; Spring, B.; Tian, L.; Domanchuk, K.; Kibbe, M.; Zhao, L.; et al. Unsupervised Exercise and Mobility Loss in Peripheral Artery Disease: A Randomized Controlled Trial. J. Am. Heart Assoc. 2015, 4, e001659.
- McDermott, M.M.; Guralnik, J.M.; Criqui, M.H.; Ferrucci, L.; Zhao, L.; Liu, K.; Domanchuk, K.; Spring, B.; Tian, L.; Kibbe, M.; et al. Home-Based Walking Exercise in Peripheral Artery Disease: 12-Month Follow-up of the GOALS Randomized Trial. J. Am. Heart Assoc. 2014, 3, e000711.
- Calabrese, M.; Garofano, M.; Palumbo, R.; Di Pietro, P.; Izzo, C.; Damato, A.; Venturini, E.; Iesu, S.; Virtuoso, N.; Strianese, A.; et al. Exercise Training and Cardiac Rehabilitation in COVID-19 Patients with Cardiovascular Complications: State of Art. Life 2021, 11, 259.
- Collins, T.C.; Lunos, S.; Carlson, T.; Henderson, K.; Lightbourne, M.; Nelson, B.; Hodges, J.S. Effects of a Home-Based Walking Intervention on Mobility and Quality of Life in People with Diabetes and Peripheral Arterial Disease: A Randomized Controlled Trial. Diabetes Care 2011, 34, 2174–2179.
- Lamberti, N.; Straudi, S.; Manfredini, R.; De Giorgi, A.; Gasbarro, V.; Zamboni, P.; Manfredini, F. Don’t Stop Walking: The in-Home Rehabilitation Program for Peripheral Artery Disease Patients during the COVID-19 Pandemic. Intern. Emerg. Med. 2021, 16, 1307–1315.
- Allen, T.J. Textbook of Work Physiology: Physiological Bases of Exercise, 4th ed.; Human Kinetics: Champaign, IL, USA, 2004; Volume 56, p. 248.
- Bobbert, A.C. Physiological Comparison of Three Types of Ergometry. J. Appl. Physiol. 1960, 15, 1007–1014.
- Stenberg, J.; Astrand, P.O.; Ekblom, B.; Royce, J.; Saltin, B. Hemodynamic Response to Work with Different Muscle Groups, Sitting and Supine. J. Appl. Physiol. 1967, 22, 61–70.
- Treat-Jacobson, D.; McDermott, M.M.; Bronas, U.G.; Campia, U.; Collins, T.C.; Criqui, M.H.; Gardner, A.W.; Hiatt, W.R.; Regensteiner, J.G.; Rich, K.; et al. Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e10–e33.
- Bronas, U.G.; Treat-Jacobson, D.; Leon, A.S. Comparison of the Effect of Upper Body-Ergometry Aerobic Training vs Treadmill Training on Central Cardiorespiratory Improvement and Walking Distance in Patients with Claudication. J. Vasc. Surg. 2011, 53, 1557–1564.
- Tew, G.; Nawaz, S.; Zwierska, I.; Saxton, J.M. Limb-Specific and Cross-Transfer Effects of Arm-Crank Exercise Training in Patients with Symptomatic Peripheral Arterial Disease. Clin. Sci. 2009, 117, 405–413.
- Lauret, G.J.; Fakhry, F.; Fokkenrood, H.J.P.; Hunink, M.G.M.; Teijink, J.A.W.; Spronk, S. Modes of Exercise Training for Intermittent Claudication. Cochrane Database Syst. Rev. 2014, 4, CD009638.
- Walker, R.D.; Nawaz, S.; Wilkinson, C.H.; Saxton, J.M.; Pockley, A.G.; Wood, R.F.M. Influence of Upper- and Lower-Limb Exercise Training on Cardiovascular Function and Walking Distances in Patients with Intermittent Claudication. J. Vasc. Surg. 2000, 31, 662–669.
- McGuigan, M.R.; Bronks, R.; Newton, R.U.; Sharman, M.J.; Graham, J.C.; Cody, D.V.; Kraemer, W.J. Resistance Training in Patients with Peripheral Arterial Disease: Effects on Myosin Isoforms, Fiber Type Distribution, and Capillary Supply to Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B302–B310.
- Sanderson, B.; Askew, C.; Stewart, I.; Walker, P.; Gibbs, H.; Green, S. Short-Term Effects of Cycle and Treadmill Training on Exercise Tolerance in Peripheral Arterial Disease. J. Vasc. Surg. 2006, 44, 119–127.
- Gomes, A.P.F.; Correia, M.A.; Soares, A.H.G.; Cucato, G.G.; Lima, A.H.R.A.; Cavalcante, B.R.; Sobral-Filho, D.C.; Ritti-Dias, R.M. Effects of Resistance Training on Cardiovascular Function in Patients With Peripheral Artery Disease: A Randomized Controlled Trial. J. Strength Cond. Res. 2018, 32, 1072–1080.
- Hiatt, W.R.; Wolfel, E.E.; Meier, R.H.; Regensteiner, J.G. Superiority of Treadmill Walking Exercise versus Strength Training for Patients with Peripheral Arterial Disease. Implications for the Mechanism of the Training Response. Circulation 1994, 90, 1866–1874.
- Ritti-Dias, R.M.; Wolosker, N.; de Moraes Forjaz, C.L.; Carvalho, C.R.F.; Cucato, G.G.; Leão, P.P.; de Fátima Nunes Marucci, M. Strength Training Increases Walking Tolerance in Intermittent Claudication Patients: Randomized Trial. J. Vasc. Surg. 2010, 51, 89–95.
- Parmenter, B.J.; Raymond, J.; Dinnen, P.; Lusby, R.J.; Fiatarone Singh, M.A. High-Intensity Progressive Resistance Training Improves Flat-Ground Walking in Older Adults with Symptomatic Peripheral Arterial Disease. J. Am. Geriatr. Soc. 2013, 61, 1964–1970.
More
