Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Vicky Zhou and Version 4 by Vicky Zhou.
Bone is one of the most common metastatic sites among breast cancer (BC) patients. Once bone metastasis is developed, patients’ survival and quality of life will be significantly declined. At present, there are limited therapeutic options for BC patients with bone metastasis. Different nanotechnology-based delivery systems have been developed aiming to specifically deliver the therapeutic agents to the bone. The conjugation of targeting agents to nanoparticles can enhance the selective delivery of various payloads to the metastatic bone lesion.
- breast cancer
- bone metastasis
- targeted drug delivery system
- nanomedicine
- nanotechnology
1. Introduction
Breast cancer (BC) is the most common malignant tumour among women [1]. According to the American Cancer Society (ACS) reports, BC leads to the second-highest cancer-related deaths in women after lung cancer. One in 38 women (about 2.6%) will die from BC. There were 2.3 million new cases in 2020 that led to 685,000 deaths globally [2]. In addition, the ACS pointed out that BC’s incidence rate increased about 0.3% per year in recent years [3]. The mortality rate also increased significantly from 1990–2015 [4]. Based on the immunohistochemical expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), BC can be classified into four major subtypes: hormone receptor (HR)+/HER2+, HR+/HER2−, HR−/HER2+ and HR-/HER2− [5][6]. In the absence of all three receptors, i.e., HR−/HER2− subtype, triple-negative breast cancer (TNBC), accounts for 15–20% of all BCs [7].
According to the molecular profiles, BC could be classified as luminal subtype, HER2 enriched+ subtype, and basal-like subtype with a high expression of basal markers [8]. The luminal subtype could be divided into luminal A and luminal B tumours. The luminal A that comprises 40% of all subtypes shows the best clinical prognosis with a high level of ER expression. Therefore, these patients are more likely to benefit from hormonal therapy alone. The other less common subtype, luminal B (20%) tumours, express ERs at a lower level but exhibit higher levels of proliferation-related genes. Consequently, patients within this category may need chemotherapy [9]. HER2 enriched tumours (15%) also show overexpression of proliferation-related genes. HER2+ tumours that are ER- are classified as luminal B subtype [10]. The basal-like group is characterised by the upregulation of genes expressed by basal/myoepithelial cells. Although it has been reported that 71% of TNBC tumours were found to be basal-like and 77% basal-like tumours were triple negative, TNBC and basal-like BC are not synonyms [8]. TNBC can be further divided into six subtypes: basal-like (BL1 and BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR), and an unspecified group (UNS) [11].
Metastasis is one of the main reasons for the high mortality rate among BC patients. About 20–30% of recurrences among early BC patients are accompanied by metastatic diseases [12]. Common metastatic sites for breast cancer are bones, liver, lungs, and brain [13]. According to recent research, bone is the most common metastatic organ, while the brain is the least. In addition, different subtypes of BCs have variable likelihoods of developing metastasis. For example, the HER+ BC and TNBC are more aggressive and more likely to develop metastasis [6]. Some studies indicate that bone metastases are more frequent in HR+ subtypes than all other BC subtypes [14][15]. Bone metastasis influences the quality of patients’ life by inducing skeletal-related events (SREs), such as bone pain and tumour-induced fracture, and decreases survival [16]. The average five-year survival rates for the patients with local-regional and metastatic recurrence are 80% and 25%, respectively [17].
2. Targeting Agents for BC Bone Metastasis
In targeted drug delivery systems (DDSs), the targeting agents play vital roles. The drug can be accurately delivered to the metastatic tumour sites with targeting agents, increasing the treatment efficacy and decreasing side effects generated by some cytotoxic compounds. Various bone targeting agents include tetracycline, bisphosphonate, γ-carboxylated glutamic acids (Gla) and some amino acids (e.g., aspartic acid (Asp), and glutamic acid (Glu)), and aptamers are investigated in different cancers [18]. However, the most commonly used bone targeting agents for DDSs have involved Arginine-Glycine-Aspartic acid (RGD) peptide [19], and two drugs from bisphosphonates family, alendronate [20] and zoledronic acid [21] (Figure 1). These agents have been found to have high potential to be employed in the development of bone targeted pharmaceuticals.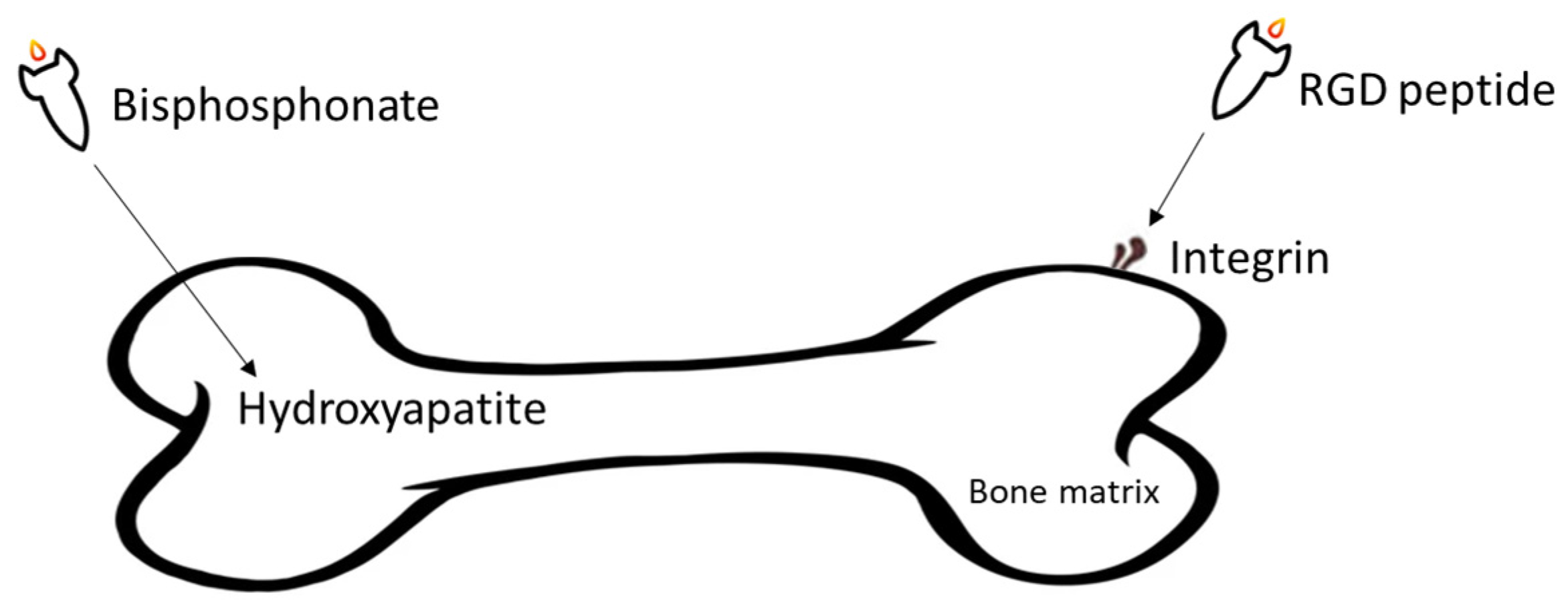
Figure 1. Overall illustration of targeting agents utilised in the development of DDSs aiming at treating BC bone metastasis.
2.1. Arginine-Glycine-Aspartic Acid (RGD) Peptide and Its Derivative
Integrins are a group of divalent cation-dependent heterodimeric membrane glycoproteins composed of α and β subunits, playing vital roles in cell-cell and cell-extracellular matrix (ECM) adhesion [22]. Among all subtypes of integrins, overexpression of ανβ3 integrin has been proven to be related to BC bone metastasis and poor prognosis and decreased survival time of BC patients [23]. As an integrin predominantly expressed in blood vessels, ανβ3 integrin can mediate angiogenesis, cell proliferation, and metastasis in several types of cancers. If ανβ3 integrin is blocked with integrin antagonists, angiogenesis of some tumour cells, such as melanoma, prostate cancer, and BC cells, would be disrupted [24][25]. Furthermore, by binding to fibronectin, fibrinogen, or osteopontin, ανβ3 integrin induces the migration of endothelial cells, and it activates several signalling cascades, which protect the cells from apoptosis [24][26].
RGD is an arginine-glycine-aspartic acid (Arg-Gly-Asp) tripeptide, which can bind specifically to ανβ3 integrin. Several preclinical studies showed that RGD peptide successfully blocked osteoclast-mediated osteolysis in bone metastatic animal models by acting as ανβ3 integrin antagonist [27]. As a peptide selectively binding to ανβ3 integrin, RGD peptide can be conjugated on drug delivery systems (DDSs) for targeted tumour therapy. Recently, RGD peptides conjugated DDSs have been widely studied in prostate cancer and bone metastasis [28][29][30]. Thus, targeting ανβ3 integrin with RGD peptide provides a promising way to treat BC metastasis.
2.2. Bisphosphonate
Bisphosphonates (BPs) are a group of chemical compounds showing high affinity to hydroxyapatite (HA) crystals commonly seen in bones and teeth. The reason for this high affinity is that BPs can generate bidentate or tridentate chelation with the calcium ion on the HA [31]. The BPs can recognise and localise quickly to tissues where HA is present after intravenous or oral administration. Thus, conjugation of BPs to the DDSs provides a promising strategy to target specifically to the bone [32]. Furthermore, BPs are widely used in the treatment of conditions where bone resorption occurs. BPs can be selectively taken up by osteoclasts [33]. By inactivating osteoclasts, they can simultaneously exert specific auxiliary therapeutic effects on SREs, such as increasing bone density, decreasing fracture risk, and relieving bone pain at the metastatic sites while playing the targeting role [34]. Different BPs can be distinguished by side-chain groups at R1 and R2 sites (Figure 2).
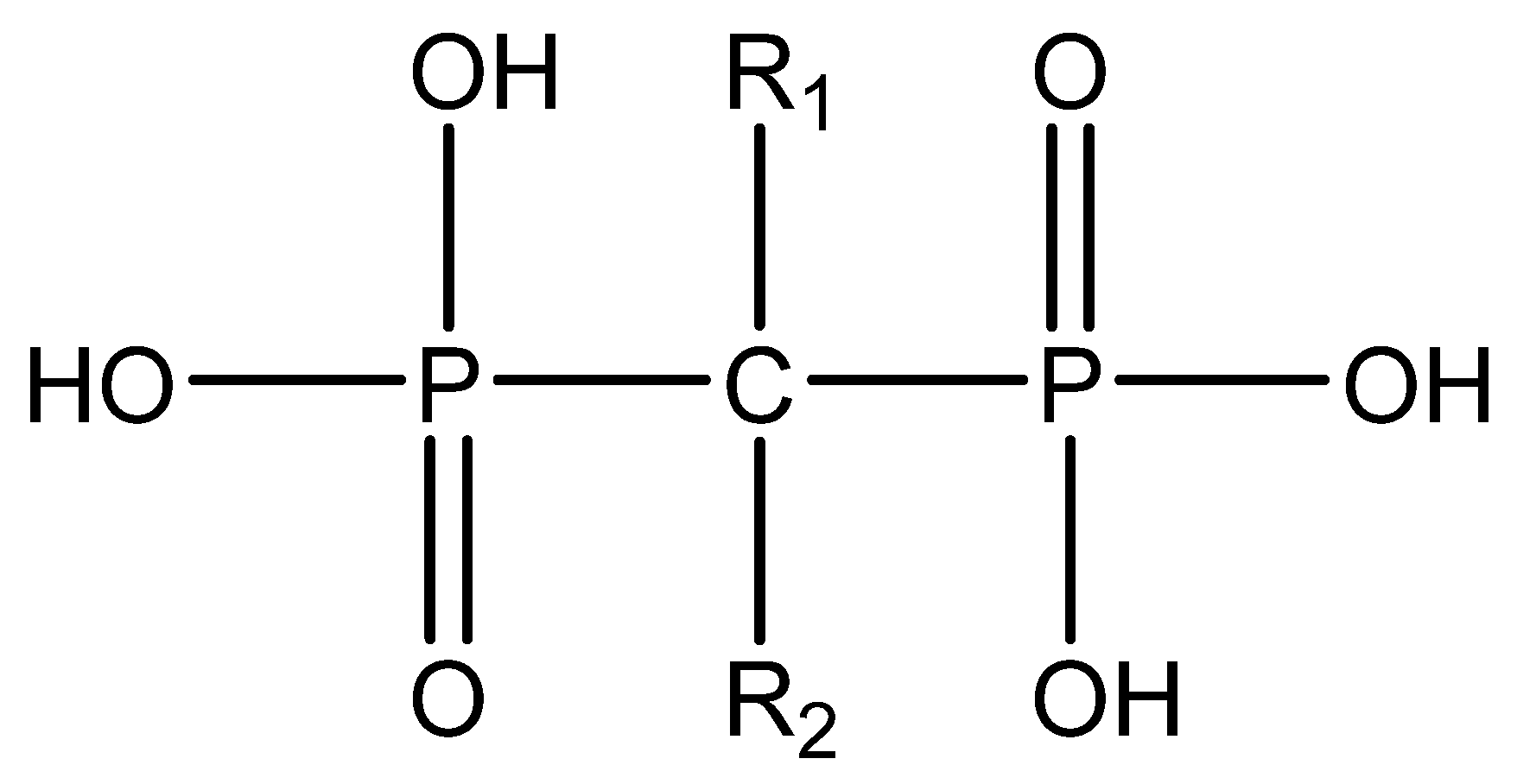
Various functional groups at R1 and R2 can affect the ability of BP to bind to HA and their antiresorptive efficacy, respectively. For example, with a hydroxyl group at the R1 side chain, the binding capacity of BPs increases significantly compared to those having a hydrogen (tiludronate) or chlorine (clodronate) on this carbon. The reason is that they can form a tridentate instead of bidentate chelation between BPs and calcium ions [35]. The antiresorptive capability of alendronate, neridronate, risedronate, olpadronate, ibandronate, zoledronic acid, and pamidronate is 10–10,000 times stronger than non-nitrogenous BPs (tiludronate, clodronate, and etidronate). This is mainly due to the presence of nitrogenous functional groups on the R2 side chain [36][37]. The results from kinetic studies of the HA crystal growth showed that the ranking of the capability of binding to HA at neat surfaces among BPs is zoledronic acid > pamidronate > alendronate > ibandronate > risedronate > etidronate > clodronate [38]. BPs from different generations and their structural differences are illustrated in Table 1.

Figure 2. The general molecular structure of bisphosphonates.
Table 1. Differences among three generations of bisphosphonates.
| Bisphosphonates | Generation | Name | R1 | R2 |
|---|---|---|---|---|
| Non-Nitrogenous | First | Etidronate | -OH | -CH3 |
| Second | Clodronate | -Cl | -Cl | |
| Tiludronate | -H | 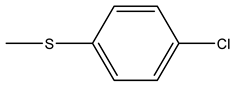 |
||
| Nitrogenous | Pamidronate | -OH |  |
|
| Third | Alendronate | -OH | 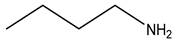 |
|
| Neridronate | -OH | 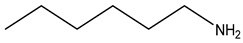 |
||
| Olpadronate | -OH | 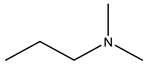 |
||
| Ibandronate | -OH | 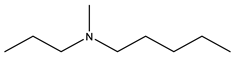 |
||
| Risedronate | -OH | 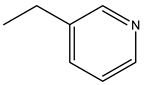 |
||
| Zoledronic acid | -OH | 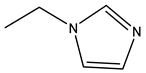 |
Besides, it has been reported that BPs could have an antitumour effect directly and indirectly among several tumours, such as prostate, lung, and melanoma cancer cells, in vitro. The potential mechanism of these antitumour activities is that BPs could mediate apoptosis and the cell cycle arrest, which suggests synergistic therapeutic effects with anticancer agents [39]. Among all BP analogues, alendronate and zoledronic acid are most frequently used as targeting agents in nanoparticles.
3. Advanced Targeted DDSs
Nanoparticles have been under investigation for a few decades because of their capability to alter the drug’s pharmacokinetics. The introduction of nanoparticles can solve the poor solubility of some hydrophobic drugs and reduce the metabolism, thus preventing the drug compounds from being degraded in the microenvironment [40][41]. The utilisation of nanoparticles for effective delivery of active pharmaceuticals could also promote the enhanced permeability and retention effect (EPR), which is widely observed in the vasculature of tissues undergoing pathologies [42]. Notably, only nanoparticles whose sizes are no more than 200 nm have the property of easily penetrating through mucus without being removed by the natural size-filtering mechanism [43].
The surface of the nanoparticles can be modified as needed to achieve specific requirements of different disease conditions [44]. Decorated with different targeting agents, the efficient delivery of various functional agents could be realised by nanoparticles. The most common targets for BC bone metastasis are HA and ανβ3 integrin. Alendronate [45] and zoledronic acid [21] are the most commonly used HA targeting agents among all BPs. The successful targeting of ανβ3 integrin is realised by involving RGD peptide into the DDSs [28][29][30]. To achieve the goal of treatment, diagnosis, and prevention of BC bone metastasis, various classes of compounds, including anticancer therapeutics [46][47], contrast agents [48], photodynamic [49], and photothermal materials [21] have been used to deliver to the bone. The following section discusses these emerging advanced DDSs depending on the nature of payloads used as described above.
3.1. Targeted DDSs with Immunostimulatory Payloads
Bisphosphonates such as zoledronic acid have also been used for the selective delivery of immunostimulatory agents. Pang and co-worker designed zoledronic acid-modified bone targeting metal-organic framework (MOF) nanoparticles loaded with immunostimulatory cytosine-phosphate-guanosine (CpG) (BT-isMOF). Both results from in vitro and in vivo studies indicated that BT-isMOF nanoparticles had a robust capability of targeting the metastatic bone lesions, leading to a significant reduction in the osteoclast-mediated bone resorption and simultaneous induction of macrophage polarisation to the M1 pro-inflammatory phenotype. This phenotype is known for the secretion of pro-inflammatory cytokines, which may also play a role in the antitumour activities [50].
3.2. Targeted DDSs Loaded with Contrast Agents
Bisphosphonate-conjugated nanoparticles loaded with contrast agents could play a role in diagnosing BC bone metastasis. Qiao et al. developed zoledronic acid-conjugated gadolinium (Ⅲ) upconversion nanoparticles (PUCZP) by encapsulating plumbagin and poly (acrylic acid) (PAA) inside bimodal mesoporous silica. With the existence of gadolinium (Ⅲ), a contrast agent in T1-MRI, PUCZP could help to detect early bone metastasis, which is generally hard to diagnose by standard radiography. With the help of PAA, the nanoparticles could release in a pH-sensitive mode in the osteoclast acidity (pH = 4.5~5.5). Furthermore, PUCZP could inhibit the expression of RANKL and further suppress the osteocyte-induced osteoclast formation and weaken the invasive properties of MDA-MB-231 and 4T1 cells in vitro. In addition, UPCZP could repress tumour growth and osteoclastogenesis in a mouse intracardiac model of BC bone metastasis [48].
3.3. Targeted DDSs Loaded with Photothermal Therapeutic Agents
Photothermal therapy (PTT) is a method that converts absorbed photon energy to heat by utilising photo-absorbing materials and killing the cancer cells under a near-infrared (NIR) laser. According to relevant studies, PTT is capable of antitumour activity by itself and could increase the sensitivity of tumour cells to anticancer compounds, which further improves the efficacy of chemotherapy [51]. In addition, PTT shows favourably non-invasive and controllable features. If combining the antitumour drugs with photothermal agents, the NIR laser could be regarded as a trigger to promote the release of the loaded drugs [52]. Zoledronic acid is widely employed in those nanoparticles designed as carriers for photothermal agents.
Nanoparticles conjugated with zoledronic acid were designed by encapsulating gold nanorods in mesoporous silica (Au@MSNs-ZOL). The Au@MSNs-ZOL showed great affinity for bone both in vitro and in vivo. In in vitro studies, the Au@MSNs-ZOL could promote the differentiation of osteoblasts and inhibit the formation of osteoclast-like cells. Furthermore, combining with NIR, Au@MSNs-ZOL could significantly reduce the volume and weight of the tumour among MDA-MB-231 bearing mice, which could relieve bone pain and reduce the bone resorption at the metastatic bone lesions [21].
Superparamagnetic iron oxide (Fe3O4) and indocyanine green (ICG)-loaded PLGA nanoparticles (ICG/Fe3O4@PLGA-ZOL) were prepared, on which zoledronic acid was conjugated as a targeting agent. In vitro, the group with magnet and laser showed less cell viability than the group without a magnet at the bottom of the plate. More nanoparticles were engulfed by the cells and killed under irradiation. In this DDS, both zoledronic acid and Fe3O4 play the targeting role, which results in a high affinity to bone and great anti-tumorigenic potency [53].
3.4. Targeted DDSs Loaded with Photodynamic Therapeutic Agents
Photodynamic therapy (PDT) is a non-invasive, safe, and selective therapeutic approach widely studied in treating various kinds of cancers. With a combination of different factors, including a photosensitiser, an appropriate wavelength, and molecular oxygen, PDT could generate reactive oxygen species (ROS), leading to cell necrosis or apoptosis [54][55]. Significantly, only cells with intracellular photosensitiser will be damaged, ensuring selectivity and safety [55].
An alendronate-functionalised DDS (BTZ@ZnPc-ALN) was developed for the selective co-delivery of bortezomib and a photosensitiser Zinc phthalocyanine to achieve the chemo-PDT of BC bone metastasis. In vivo studies showed that BTZ@ZnPc-ALN could reduce the tumour volume by 85% compared to the control group in MDA-MB-231 bearing mice, which was realised by inducing ROS-induced mitochondrial damage. In addition, the expression of GRP78 protein and the cytosolic Ca2+ levels increased, resulting in excessive endoplasmic reticulum stress leading to the inhibition of tumour cell proliferation [49]. Detailed information about targeted DDSs for prevention and treatment of BC bone metastasis is summarised in Table 2.
Table 2. A summary of targeted therapeutics for BC bone metastasis.
| Nanoparticle | Particle Size (nm) | Particle Type | Zeta Potential (mV) |
Targeting Agent | Loaded Compound |
|---|---|---|---|---|---|
| Zn@PEG-ALN NPs | About 55 * | Polymeric nanoparticle | About −25 * | Alendronate | Cisplatin prodrug |
| DZ@ALN | 61 ± 0.78 | Polymeric nanoparticle | −23.5 ± 0.41 | Alendronate | Cisplatin prodrug and Zoledronate |
| ALN-NPs | 95 ± 15 | Micelle | −11.7 ± 4.3 | Alendronate | Bortezomib |
| ALN-oHA-S-S-CUR | 179 ± 23 | Micelle | −25.7 ± 0.7 | Alendronate | Curcumin |
| ALN-oHA-S-S-CUR | 180 | Micelle | / | Alendronate | Curcumin |
| Alendronate coated PLGA nanoparticles | 235.5 ± 71.3 | Polymeric nanoparticle | / | Alendronate | Bortezomib and Curcumin |
| DOX@ALN-(HA-PASP)CL | 110 ± 9 | Polymeric nanoparticle | −16.3 ± 3.7 | Alendronate | Doxorubicin |
| NGO-ALs | 60–150 | Nanosheet | / | Alendronate | Doxorubicin |
| A1-L-DOX-Lip A10-L-DOX-Lip |
107.2 ± 4.8 106.5 ± 3.5 |
Liposome | −11.5 ± 1.96 −12.3 ± 2.01 |
Alendronate | Doxorubicin |
| ALN-PEG/C18/HYD-DOX-g-PASPAM | About 200 | Micelle | / | Alendronate | Doxorubicin |
| ALN-m/DTX | 84 ± 5 | Micelle | −30 ± 2 | Alendronate | Docetaxel |
| PMBA-DTX | 27.0 ± 0.1 | Micelle | −11.8 ± 1.6 | Alendronate | Docetaxel |
| PTX-AFTPNs (A to F ratio: 0.67) | 125.9 ± 0.95 | Polymeric nanoparticle | −29.6 ± 1.21 | Alendronate | Paclitaxel |
| Pull-(GGPNle-φ-PTX)-(PEG-ALN) | 163.3 ± 18.3 (pH = 5.5) |
Micelle | / | Alendronate | Paclitaxel |
| GANT58-BTNPs | About 100 | Micelle | / | Alendronate | Small molecule inhibitors of Gli2 |
| BTZ@ZnPc-ALN | About 60 | Polymeric nanoparticle | −18 mV | Alendronate | Bortezomib and Zinc phthalocyanine |
| Au@MSNs-ZOL | About 70 | Mesoporous silica nanoparticle | +24.3 | Zoledronic acid | Gold nanorods |
| BT-isMOF | 228 ± 12 | Metal−organic framework nanoparticle | / | Zoledronic acid | Immunostimulatory oligonucleotide |
| PBCA-PEG-ZOL NPs | 82 ± 6.35 | Polymeric nanoparticle | From −8.26 ± 1.26 to −23.51 ± 3.37 | Zoledronic acid | Docetaxel |
| UCZP | About 60 | Mesoporous silica nanoparticle | −18.9 | Zoledronic acid | Gadolinium |
| ICG/Fe3O4@PLGA-ZOL | 313.9 | Polymeric nanoparticle | −15.0 | Zoledronic acid | Iron oxide (Fe3O4) and indocyanine green |
| DPA−G5-PEG−cRGD/BTZ | 78.02 * | Polymeric nanoparticle | −3.425 * | RGD peptide | Bortezomib |
| PTX-Glu6-RGD-Lip | 121.9 ± 4.7 | Liposome | −14.37 ± 4.85 | RGD peptide (Glu6-RGD) derivative | Paclitaxel |
| αvβ3-MPs | 12.5 ± 0.8 | Micelle | −3.82 ± 1.23 | Quinolone nonpeptide | Docetaxel |
* The exact value was not given in the original paper.
4. Conclusions and Perspectives
As the most common site of metastasis, bone metastasis seriously affects BC patients’ survival rate and quality of life and remains a challenging clinical condition. Therefore, effective treatments are needed to avoid the devastating impacts of BC bone metastasis on patients. One way to achieve this could be the development of targeted DDSs. Nanotechnology-based delivery systems have shown several advantages in treating BC bone metastasis compared to the conventional therapies, including: (1) solve the challenges associated with poor physicochemical properties of the payloads such as low solubility of hydrophobic drugs; (2) increase the therapeutic index of drugs; (3) enhance the metabolic stability and plasma circulation times of the payloads; (4) promote passive targeting through the EPR effect and mediate active targeting to the bone using different targeting moieties; (5) enable triggered release of the payloads. With the conjugation of a targeting agent, these DDSs can specifically deliver payloads to the bone metastatic lesions, which can greatly reduce the side effect of the cytotoxic anticancer reagents. Herein highlighted the research involving DDSs relevant to the targeted treatment of BC bone metastasis, suggesting promising therapeutic options for this unmet condition. However, being in their early preclinical stages of development, there are still challenges for the future translation of these technologies to the clinic. Some of those include optimising the fabrication process for upscaling to achieve clinical translation and designing and conducting studies that inform about the fate of DDSs in vivo and their interaction with blood, healthy and diseased tissues, and cells, as well as intracellular compartments. Furthermore, lessons from previous drug developments in the area of nanomedicine have shown that those DDSs with more complex designs face additional challenges in their optimisation and characterisation that leads to lower reproducibility in the production processes. To fully uncover the potential of nanotechnology-based DDSs in BC bone metastasis, it is necessary to understand the nanomaterials’ properties and the metastasis itself. This allows more in-depth investigations on the interaction between these DDSs and the BC bone metastasis.
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150.
- Breast Cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 25 July 2021).
- How Common Is Breast Cancer? 2020. Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 25 July 2021).
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020.
- Sorlie, T.; Parker, J. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423.
- Wang, H.; Zhang, C.; Zhang, J.; Kong, L.; Zhu, H.; Yu, J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: A SEER based study. Oncotarget 2017, 8, 26368–26379.
- Oner, G.; Altintas, S.; Canturk, Z.; Tjalma, W.; Verhoeven, Y.; Van Berckelaer, C.; Berneman, Z.; Peeters, M.; Pauwels, P.; van Dam, P.A. Triple-negative breast cancer-Role of immunology: A systemic review. Breast J. 2020, 26, 995–999.
- Alluri, P.; Newman, L.A. Basal-like and triple-negative breast cancers: Searching for positives among many negatives. Surg. Oncol. Clin. N. Am. 2014, 23, 567–577.
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35.
- Provenzano, E.; Ulaner, G.A.; Chin, S.F. Molecular Classification of Breast Cancer. PET Clin. 2018, 13, 325–338.
- Wang, D.Y.; Jiang, Z.; Ben-David, Y.; Woodgett, J.R.; Zacksenhaus, E. Molecular stratification within triple-negative breast cancer subtypes. Sci. Rep. 2019, 9, 19107.
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277.
- Gerratana, L.; Fanotto, V.; Bonotto, M.; Bolzonello, S.; Minisini, A.M.; Fasola, G.; Puglisi, F. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 2015, 32, 125–133.
- Beca, F.; Santos, R.; Vieira, D.; Zeferino, L.; Dufloth, R.; Schmitt, F. Primary relapse site pattern in women with triple-negative breast cancer. Pathol. Res. Pract. 2014, 210, 571–575.
- Sihto, H.; Lundin, J.; Lundin, M.; Lehtimaki, T.; Ristimaki, A.; Holli, K.; Sailas, L.; Kataja, V.; Turpeenniemi-Hujanen, T.; Isola, J.; et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: A nationwide cohort study. Breast Cancer Res. BCR 2011, 13, R87.
- Zhang, H.; Zhu, W.; Biskup, E.; Yang, W.; Yang, Z.; Wang, H.; Qiu, X.; Zhang, C.; Hu, G.; Hu, G. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal-related events (SREs) in breast cancer patients: A systematic review of the real world data. J. Bone Oncol. 2018, 11, 38–50.
- Odle, T.G. Precision Medicine in Breast Cancer. Radiol. Technol. 2017, 88, 401M–421M.
- Katsumi, H.; Yamashita, S.; Morishita, M.; Yamamoto, A. Bone-Targeted Drug Delivery Systems and Strategies for Treatment of Bone Metastasis. Chem. Pharm. Bull. 2020, 68, 560–566.
- Wang, M.; Cai, X.; Yang, J.; Wang, C.; Tong, L.; Xiao, J.; Li, L. A Targeted and pH-Responsive Bortezomib Nanomedicine in the Treatment of Metastatic Bone Tumors. ACS Appl. Mater. Interfaces 2018, 10, 41003–41011.
- Liu, T.; Romanova, S.; Wang, S.; Hyun, M.A.; Zhang, C.; Cohen, S.M.; Singh, R.K.; Bronich, T.K. Alendronate-Modified Polymeric Micelles for the Treatment of Breast Cancer Bone Metastasis. Mol. Pharm. 2019, 16, 2872–2883.
- Sun, W.; Ge, K.; Jin, Y.; Han, Y.; Zhang, H.; Zhou, G.; Yang, X.; Liu, D.; Liu, H.; Liang, X.J.; et al. Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano 2019, 13, 7556–7567.
- Foubert, P.; Varner, J.A. Integrins in tumor angiogenesis and lymphangiogenesis. Methods Mol. Biol. 2012, 757, 471–486.
- Wang, L.; Song, L.; Li, J.; Wang, Y.; Yang, C.; Kou, X.; Xiao, B.; Zhang, W.; Li, L.; Liu, S.; et al. Bone sialoprotein-alphavbeta3 integrin axis promotes breast cancer metastasis to the bone. Cancer Sci. 2019, 110, 3157–3172.
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164.
- Sloan, E.K.; Pouliot, N.; Stanley, K.L.; Chia, J.; Moseley, J.M.; Hards, D.K.; Anderson, R.L. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. BCR 2006, 8, R20.
- Danhier, F.; Le Breton, A.; Preat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973.
- Schneider, J.G.; Amend, S.R.; Weilbaecher, K.N. Integrins and bone metastasis: Integrating tumor cell and stromal cell interactions. Bone 2011, 48, 54–65.
- Zhang, X.; He, Z.; Xiang, L.; Li, L.; Zhang, H.; Lin, F.; Cao, H. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Des. Dev. Ther. 2019, 13, 1357–1372.
- Sutherland, M.; Gordon, A.; Shnyder, S.D.; Patterson, L.H.; Sheldrake, H.M. RGD-Binding Integrins in Prostate Cancer: Expression Patterns and Therapeutic Prospects against Bone Metastasis. Cancers 2012, 4, 1106–1145.
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233.
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19.
- Pignatello, R.; Cenni, E.; Micieli, D.; Fotia, C.; Salerno, M.; Granchi, D.; Avnet, S.; Sarpietro, M.G.; Castelli, F.; Baldini, N. A novel biomaterial for osteotropic drug nanocarriers: Synthesis and biocompatibility evaluation of a PLGA–ALE conjugate. Nanomedicine 2009, 4, 161–175.
- Bai, S.B.; Liu, D.Z.; Cheng, Y.; Cui, H.; Liu, M.; Cui, M.X.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Osteoclasts and tumor cells dual targeting nanoparticle to treat bone metastases of lung cancer. Nanomedicine 2019, 21, 102054.
- Celin, M.R.; Simon, J.C.; Krzak, J.J.; Fial, A.V.; Kruger, K.M.; Smith, P.A.; Harris, G.F. Do Bisphosphonates Alleviate Pain in Children? A Systematic Review. Curr. Osteoporos Rep. 2020, 18, 486–504.
- Russell, R.G. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007, 119 (Suppl. 2), S150–S162.
- George, S.; Weber, D.R.; Kaplan, P.; Hummel, K.; Monk, H.M.; Levine, M.A. Short-Term Safety of Zoledronic Acid in Young Patients with Bone Disorders: An Extensive Institutional Experience. J. Clin. Endocrinol. Metab. 2015, 100, 4163–4171.
- Hampson, G.; Fogelman, I. Clinical role of bisphosphonate therapy. Int. J. Women’s Health 2012, 4, 455–469.
- Lin, T.-J. Predicting binding affinities of nitrogen-containing bisphosphonates on hydroxyapatite surface by molecular dynamics. Chem. Phys. Lett. 2019, 716, 83–92.
- Benyettou, F.; Lalatonne, Y.; Sainte-Catherine, O.; Monteil, M.; Motte, L. Superparamagnetic nanovector with anti-cancer properties: Gamma Fe2O3@Zoledronate. Int. J. Pharm. 2009, 379, 324–327.
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H.J.P.R. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037.
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 9, 2539–2555.
- Forrest, M.L.; Kwon, G.S. Clinical developments in drug delivery nanotechnology. Adv. Drug Deliv. Rev. 2008, 60, 861–862.
- Lababidi, N.; Sigal, V.; Koenneke, A.; Schwarzkopf, K.; Manz, A.; Schneider, M. Microfluidics as tool to prepare size-tunable PLGA nanoparticles with high curcumin encapsulation for efficient mucus penetration. Beilstein J. Nanotechnol. 2019, 10, 2280–2293.
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158.
- Chen, S.H.; Liu, T.I.; Chuang, C.L.; Chen, H.H.; Chiang, W.H.; Chiu, H.C. Alendronate/folic acid-decorated polymeric nanoparticles for hierarchically targetable chemotherapy against bone metastatic breast cancer. J. Mater. Chem. B 2020, 8, 3789–3800.
- Ye, W.L.; Zhao, Y.P.; Li, H.Q.; Na, R.; Li, F.; Mei, Q.B.; Zhao, M.G.; Zhou, S.Y. Doxorubicin-poly (ethylene glycol)-alendronate self-assembled micelles for targeted therapy of bone metastatic cancer. Sci. Rep. 2015, 5, 14614.
- Lim, C.W.; Kim, D. Bone targeting nano-aggregates prepared from self-assembled polyaspartamide graft copolymers for pH sensitive DOX delivery. Biomater. Sci. 2021, 9, 1660–1667.
- Qiao, H.; Cui, Z.; Yang, S.; Ji, D.; Wang, Y.; Yang, Y.; Han, X.; Fan, Q.; Qin, A.; Wang, T.; et al. Targeting Osteocytes to Attenuate Early Breast Cancer Bone Metastasis by Theranostic Upconversion Nanoparticles with Responsive Plumbagin Release. ACS Nano 2017, 11, 7259–7273.
- Huang, Y.; Xiao, Z.; Guan, Z.; Shen, Y.; Jiang, Y.; Xu, X.; Huang, Z.; Zhao, C. A light-triggered self-reinforced nanoagent for targeted chemo-photodynamic therapy of breast cancer bone metastases via ER stress and mitochondria mediated apoptotic pathways. J. Control. Release 2020, 319, 119–134.
- Pang, Y.; Fu, Y.; Li, C.; Wu, Z.; Cao, W.; Hu, X.; Sun, X.; He, W.; Cao, X.; Ling, D.; et al. Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer-Associated Osteolysis. Nano Lett. 2020, 20, 829–840.
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895.
- Cai, S.; Yan, J.; Xiong, H.; Xing, H.; Liu, Y.; Liu, S.; Liu, Z. Aptamer-Functionalized Molybdenum Disulfide Nanosheets for Tumor Cell Targeting and Lysosomal Acidic Environment/NIR Laser Responsive Drug Delivery to Realize Synergetic Chemo-Photothermal Therapeutic Effects. Int. J. Pharm. 2020, 590, 119948.
- Jiang, Z.; Li, J.; Chen, S.; Guo, Q.; Jing, Z.; Huang, B.; Pan, Y.; Wang, L.; Hu, Y. Zoledronate and SPIO dual-targeting nanoparticles loaded with ICG for photothermal therapy of breast cancer tibial metastasis. Sci. Rep. 2020, 10, 13675.
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107.
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425.
More
