Graphene is a two-dimensional (2D) material composed of sp2 carbon monolayer arranged into a hexagonal network. Reduced graphene oxide (rGO), a carbon nanostructure from the graphene derivatives family, has been incorporated in composite materials due to its remarkable electrical conductivity, mechanical strength capacity, and low cost. Graphene oxide (GO) is typically synthesized by the improved Hummers’ method and then chemically reduced to obtain rGO.
- reduced graphene oxide
- clays
- hydrothermal carbons
- supported carbons
- polymer composites
1. Introduction
2. Chemical Reduction of Graphene Oxide
rGO is the most used 2D carbon material for the development of electrically conductive and mechanically reinforced polymer composites [3]. Graphite, constituted by graphene layers bonded by strong van der Waals forces, is the bulk starting material to synthesize rGO. First, graphite is oxidized to produce GO. After that, GO suffers a reduction step to produce rGO, as shown in Figure 1.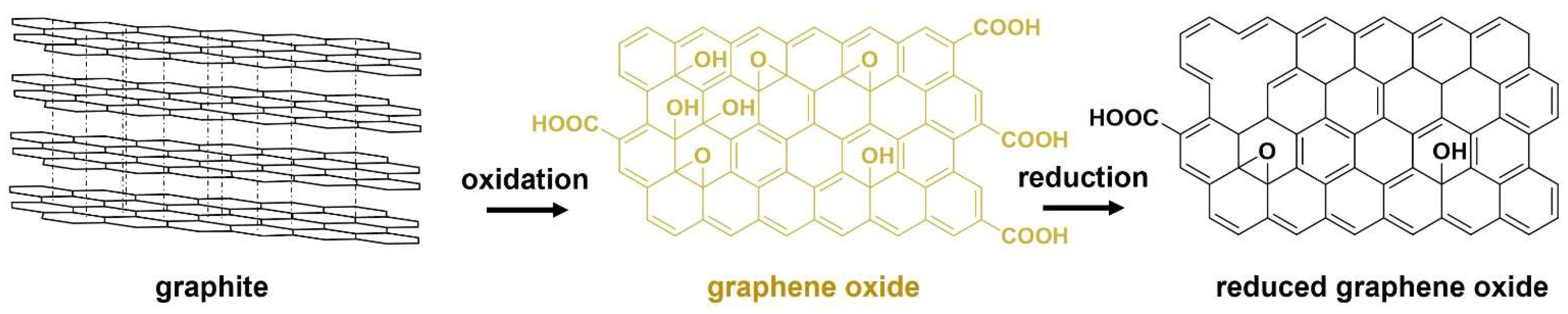
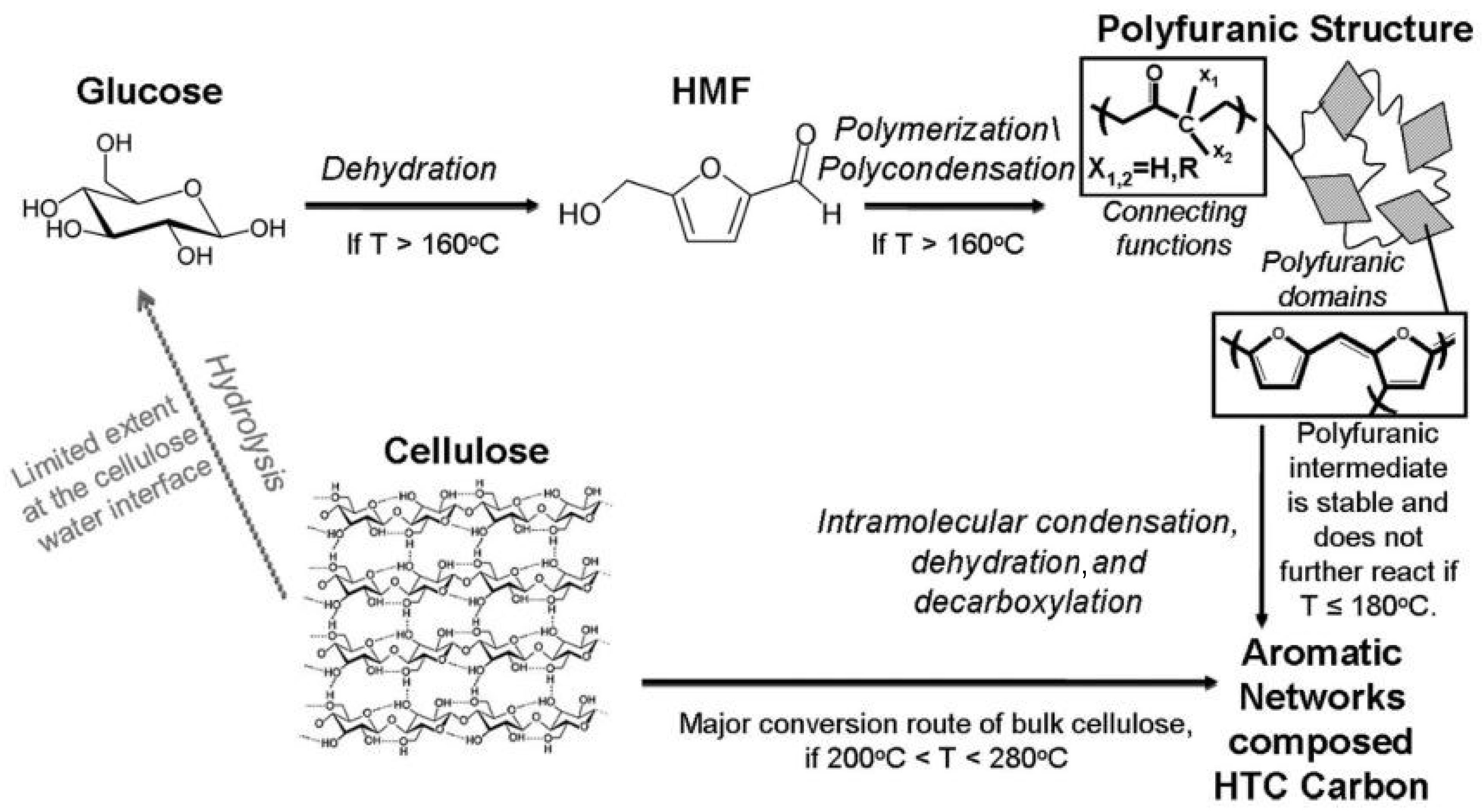
3. Carbon Structures Derived from Biomass
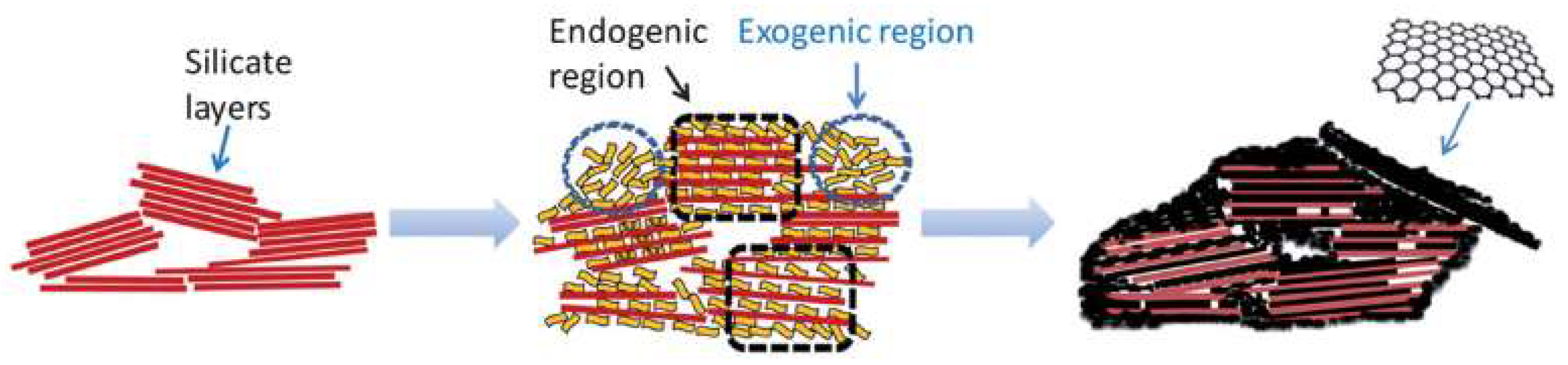
4. Graphitic Materials Supported on Lamellar Structures
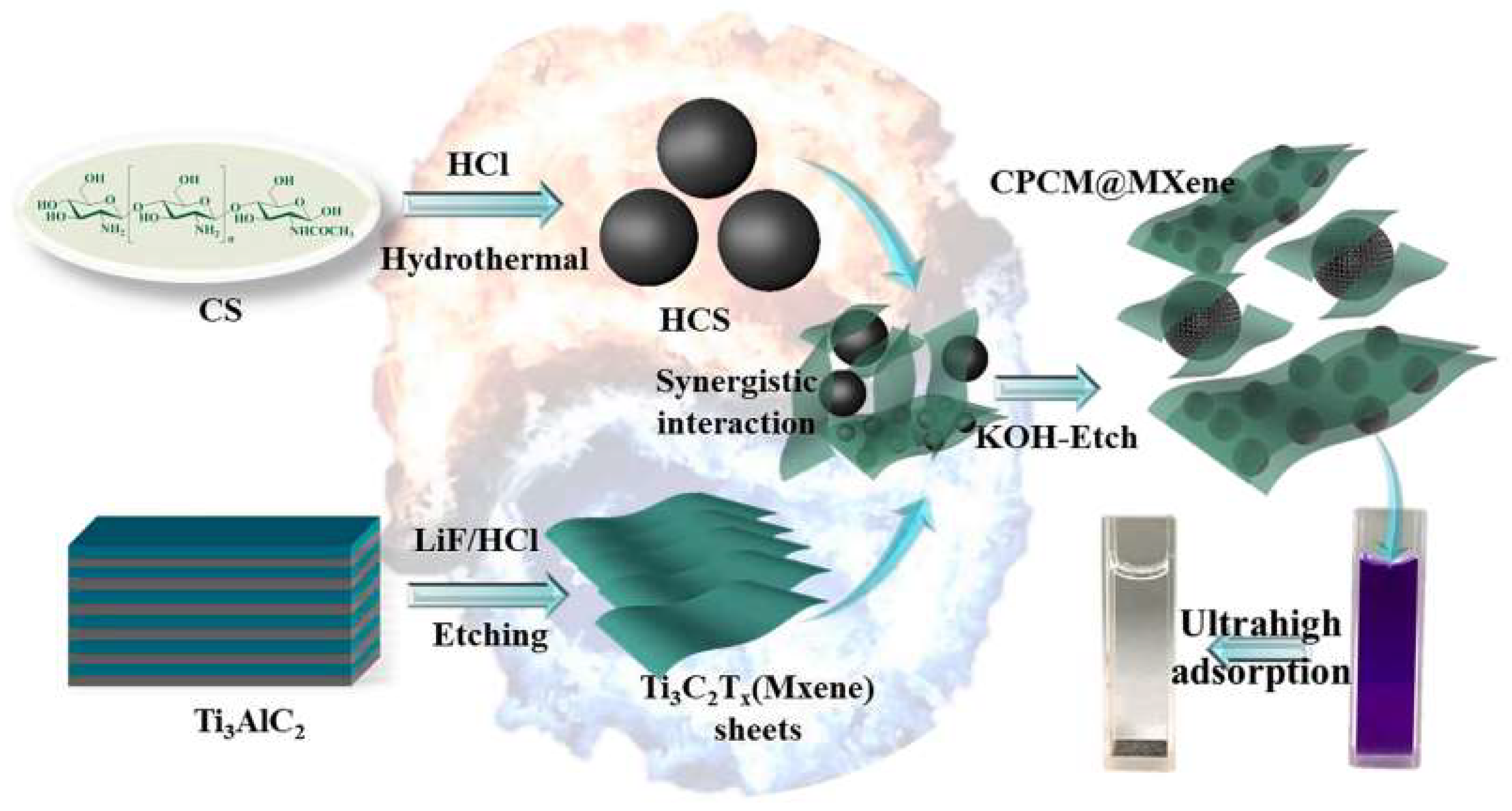
5. Polymer Composites Containing rGO
Application | Carbon Nanostructure | Polymer Composite | Results | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Corrosion protection | rGO (Urtica dioica leaf) | Polyurethane/rGO (0.15 wt%) coatings (tested on mild steel) | Resistance against accelerated weathering condition; improved UV shielding and corrosion protection efficiency. | [49] | ||||||||||||
Corrosion protection | rGO (Peganum harmala seed) | Epoxy resin/rGO-Zn (0.15 wt%) coatings (tested on steel) | Dual active and barrier corrosion protection. | [50] | ||||||||||||
Corrosion protection | rGO (Peganum harmala seed) | Epoxy ester resin/rGO-Zn (0.15 wt%) coating (tested on steel) | Improved tensile strength (78%), Young’s modulus (102%) and fracture energy (83%); improved thermal stability (62%). | [51] | ||||||||||||
Gas diffusion barrier | rGO (elemental sulfur) | Polyimide/rGO (0.5–5 wt%) films | Improved tensile strength and Young’s modulus; 95% reduction of oxygen permeability. | [52] | ||||||||||||
Sensing | rGO (Bougainvillea glabra flower) | Nafion/rGO solution drop-casted on a carbon working electrode | Sensor electrode used for Pb2+ detection; improved sensitivity and ultralow limit of detection. | [53] | ||||||||||||
Supercapacitors | rGO (eucalyptus bark) | Nafion/rGO solution drop-casted on a glassy carbon electrode | High specific capacitance (239 F g−1) and high energy density (71 W h kg−1) at a current density of 2 A g−1. | [54] | ||||||||||||
Environmental remediation | rGO (Pseudoalteromonas sp.) | Sodium alginate/rGO solution dripped into CaCl2 solution to obtain spheres | MB and CR dye adsorption from water. Reusable absorbent with adsorption efficiency of the MB and CR 77.91% and 68.27% after 4 adsorption–desorption cycles. | [55] | ||||||||||||
Food packaging | rGO (HTC/caffeic acid) | Chitosan/rGO (50%) film | Electrically conductive film to sterilize food by in-pack PEF; electrical conductivity of 0.7 S m−1 and 2.1 × 10−5 S m−1 in-plane and through-plane, respectively. | [9] | ||||||||||||
Food packaging | rGO (HTC/ZnO) | Alginate/sepiolite/ZnO-rGO (50%) | Antimicrobial and electrically conductive film for food packaging. E. coli and S. | Inhibition of aureus growth; electrical conductivity of 0.1 S m−1 and 7.5 × 10−5 S m−1 in-plane and through-plane, respectively. | [56] | |||||||||||
Not mentioned | rGO (BM/Zn) | Epoxy resin/rGO (0.1–0.3%) composites | Improvement of thermomechanical properties. | [58] |
rGO: reduced graphene oxide. UV: ultraviolet. MB: methylene blue. CR: Congo red. HTC: hydrothermal carbonization. PEF: pulsed electric field. BM: ball milling.
References
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat. Rev. Chem. 2018, 2, 0100.
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the Gap between Reality and Ideal in Chemical Vapor Deposition Growth of Graphene. Chem. Rev. 2018, 118, 9281–9343.
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S. Graphene-Based ultracapacitors. Nano Lett. 2008, 8, 3498–3502.
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials; Wiley-VCH Verlag: New York, NY, USA, 2014; Volume 53, pp. 7714–7718.
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of amphiphilic graphene nanoplatelets. Small 2009, 5, 82–85.
- Loeffen, A.; Cree, D.E.; Sabzevari, M.; Wilson, L.D. Effect of Graphene Oxide as a Reinforcement in a Bio-Epoxy Composite. J. Compos. Sci. 2021, 5, 91.
- Barra, A.; Ferreira, N.M.N.M.; Martins, M.A.M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.A.A.; Neumayer, S.M.S.M.; Rodriguez, B.J.B.J.; Enăchescu, M.; Ferreira, P.; et al. Eco-friendly preparation of electrically conductive chitosan-reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60.
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393.
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814.
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857.
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290.
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854.
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401.
- Kazmierczak-Razna, J.; Nowicki, P.; Wiśniewska, M.; Nosal-Wiercińska, A.; Pietrzak, R. Thermal and physicochemical properties of phosphorus-containing activated carbons obtained from biomass. J. Taiwan Inst. Chem. Eng. 2017, 80, 1006–1013.
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; White, R.J. Carbonaceous Materials. U.S. Patent 8790548B2, 29 July 2014.
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New Starch-Derived Mesoporous Carbonaceous Materials with Tunable Properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786.
- White, R.J.; Antonio, C.; Budarin, V.L.; Bergström, E.; Thomas-Oates, J.; Clark, J.H. Polysaccharide-derived carbons for polar analyte separations. Adv. Funct. Mater. 2010, 20, 1834–1841.
- Jung, A.; Han, S.; Kim, T.; Cho, W.J.; Lee, K. Synthesis of high carbon content microspheres using 2-step microwave carbonization, and the influence of nitrogen doping on catalytic activity. Carbon N. Y. 2013, 60, 307–316.
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273.
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212.
- Falco, C.; Caballero, F.P.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.-M.; Baccile, N. Hydrothermal Carbon from Biomass: Structural Differences between Hydrothermal and Pyrolyzed Carbons via 13C Solid State NMR. Langmuir 2011, 27, 14460–14471.
- Krishnan, D.; Raidongia, K.; Shao, J.; Huang, J. Graphene oxide assisted hydrothermal carbonization of carbon hydrates. ACS Nano 2014, 8, 449–457.
- Li, Y.Q.; Samad, Y.A.; Polychronopoulou, K.; Liao, K. Lightweight and Highly Conductive Aerogel-like Carbon from Sugarcane with Superior Mechanical and EMI Shielding Properties. ACS Sustain. Chem. Eng. 2015, 3, 1419–1427.
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787.
- Zhong, R.; Liao, Y.; Shu, R.; Ma, L.; Sels, B.F. Vapor-phase assisted hydrothermal carbon from sucrose and its application in acid catalysis. Green Chem. 2018, 20, 1345–1353.
- Yu, L.; Falco, C.; Weber, J.; White, R.J.; Howe, J.Y.; Titirici, M.M. Carbohydrate-derived hydrothermal carbons: A thorough characterization study. Langmuir 2012, 28, 12373–12383.
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.; Kruse, A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 260, 121101.
- Ruiz-Hitzky, E.; Aranda, P.; Serratosa, J.M. Clay-organic Interactions: Organoclay Complexes and Polymer-clay Nanocomposite. In Handbook of Layered Materials; Aucherbach, S., Carrado, K.A., Dutta, P., Eds.; Taylor & Francis: New York, NY, USA, 2004; p. 19.
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; ISBN 9780080441832.
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158.
- Kyotani, T.; Sonobe, N.; Tomita, A. Formation of highly orientated graphite from polyacrylonitrile by using a two-dimensional space between montmorillonite lamellae. Nature 1988, 331, 331–333.
- Fernández-Saavedra, R.; Aranda, P.; Ruiz-Hitzky, E. Templated synthesis of carbon nanofibers from polyacrylonitrile using sepiolite. Adv. Funct. Mater. 2004, 14, 77–82.
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Zatile, E.; Palomares, F.J.; Aranda, P. Supported graphene from natural resources: Easy preparation and applications. Adv. Mater. 2011, 23, 5250–5255.
- Darder, M.; Ruiz-Hitzky, E. Caramel-clay nanocomposites. J. Mater. Chem. 2005, 15, 3913–3918.
- Ruiz-Hitzky, E. Molecular access to intracrystalline tunnels of sepiolite. J. Mater. Chem. 2001, 11, 86–91.
- Wu, X.; Zhang, Q.; Liu, C.; Zhang, X.; Chung, D.D.L. Carbon-coated sepiolite clay fibers with acid pre-treatment as low-cost organic adsorbents. Carbon 2017, 123, 259–272.
- Yebra-Rodríguez, A.; Martín-Ramos, J.D.; Del Rey, F.; Viseras, C.; López-Galindo, A. Effect of acid treatment on the structure of sepiolite. Clay Miner. 2003, 38, 353–360.
- Aznar, A.J.; Sanz, J.; Ruiz-Hitzky, E. Mechanism of the grafting of organosilanes on mineral surfaces. IV. Phenylderivatives of sepiolite and poly (organosiloxanes). Colloid Polym. Sci. 1992, 270, 165–176.
- Gozález, L.; Ibarra, L.M.; Rodríguez, A.; Moya, J.S.; Valle, F.J. Fibrous silica gel obtained from sepiolite by HCl attack. Clay Miner. 1984, 19, 93–98.
- Ruiz-García, C.; Pérez-Carvajal, J.; Berenguer-Murcia, A.; Darder, M.; Aranda, P.; Cazorla-Amorós, D.; Ruiz-Hitzky, E. Clay-supported graphene materials: Application to hydrogen storage. Phys. Chem. Chem. Phys. 2013, 15, 18635–18641.
- Ruiz-García, C.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Toward a green way for the chemical production of supported graphenes using porous solids. J. Mater. Chem. A 2017, 2009–2017.
- Liu, C.; Cai, W.; Liu, L. Applied Clay Science Hydrothermal carbonization synthesis of Al-pillared composites as high performing toluene adsorbents. Appl. Clay Sci. 2018, 162, 113–120.
- Gabriel, R.; Gonçalves, L.; De Carvalho, M.; Morais, R.; Andrade, E.; Frigi, G.; Macedo, S.; Augusto, M.; Regina, V.; Constantino, L.; et al. Mesoporous carbon derived from a biopolymer and a clay: Preparation, characterization and application for an organochlorine pesticide adsorption. Microporous Mesoporous Mater. 2016, 225, 342–354.
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131.
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779.
- Wu, Z.; Deng, W.; Tang, S.; Ruiz-Hitzky, E.; Luo, J.; Wang, X. Pod-inspired MXene/porous carbon microspheres with ultrahigh adsorption capacity towards crystal violet. Chem. Eng. J. 2021, 426, 130776.
- Mahmudzadeh, M.; Yari, H.; Ramezanzadeh, B.; Mahdavian, M. Urtica dioica extract as a facile green reductant of graphene oxide for UV resistant and corrosion protective polyurethane coating fabrication. J. Ind. Eng. Chem. 2019, 78, 125–136.
- Mohammadkhani, R.; Ramezanzadeh, M.; Akbarzadeh, S.; Bahlakeh, G.; Ramezanzadeh, B. Graphene oxide nanoplatforms reduction by green plant-sourced organic compounds for construction of an active anti-corrosion coating; experimental/electronic-scale DFT-D modeling studies. Chem. Eng. J. 2020, 397, 125433.
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Green synthesis of reduced graphene oxide nanosheets decorated with zinc-centered metal-organic film for epoxy-ester composite coating reinforcement: DFT-D modeling and experimental explorations. J. Taiwan Inst. Chem. Eng. 2020, 114, 311–330.
- Nam, K.H.; Kim, K.; Kim, S.G.; Lee, H.S.; Jung, H.; Yu, J.; Jang, S.G.; Ku, B.C.; Moon, B.; You, N.H. Sustainable production of reduced graphene oxide using elemental sulfur for multifunctional composites. Compos. Part B Eng. 2019, 176, 107236.
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356.
- Manchala, S.; Tandava, V.S.R.K.; Jampaiah, D.; Bhargava, S.K.; Shanker, V. Novel and Highly Efficient Strategy for the Green Synthesis of Soluble Graphene by Aqueous Polyphenol Extracts of Eucalyptus Bark and Its Applications in High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11612–11620.
- Xu, B.; Cheng, S.; Han, M.; Li, Q.; Chen, W.; Zhou, W. The characteristic and performance of reduced graphene oxide by marine bacterium Pseudoalteromonas sp. CF10-13. Ceram. Int. 2020, 46, 21699–21706.
- Alves, Z.; Ferreira, N.M.; Mendo, S.; Ferreira, P.; Nunes, C. Design of Alginate-Based Bionanocomposites with Electrical Conductivity for Active Food Packaging. Int. J. Mol. Sci. 2021, 22, 9943.
- Barra, A.; Santos, J.D.C.; Silva, M.R.F.; Nunes, C.; Ruiz-Hitzky, E.; Gonçalves, I.; Yildirim, S.; Ferreira, P.; Marques, P.A.A.P. Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications. Nanomaterials 2020, 10, 2077.
- Tiwari, S.K.; Nimbalkar, A.S.; Hong, C.K.; Ha, S.K. A Green Route for Quick and Kilogram Production of Reduced Graphene Oxide and Their Applications at Low Loadings in Epoxy Resins. ChemistrySelect 2019, 4, 1266–1274.
