Surgical and locoregional treatments of peritoneal metastasis have gained increasing acceptance. Apart from systemic chemotherapy and surgical removal of the tumor, locoregional therapies such as cytoreductive surgery (CRS/HIPEC or PIPAC)/hyperthermic intraperitoneal chemotherapy (HIPEC) or pressurized intraperitoneal aerosol chemotherapy (PIPAC) may improve tumor control.
- peritoneal metastasis
- colorectal cancer (CRC)
- cytoreductive surgery
- hyperthermic intraperitoneal chemotherapy
1. CRS and HIPEC, a Locoregional Treatment Approach for Peritoneal Metastasis
Although the surgical concepts for cytoreduction are standardized, no standardization has been established thus far for HIPEC protocols with regard to temperature, treatment duration or the type of drug. Current HIPEC protocols for patients with colorectal PM therefore lack consistency and differ among countries and hospitals. For example, one proposed treatment protocol is the use of heated Oxaliplatin (Oxa), a third-generation platinum forming intra- and interstrand crosslinks in the DNA, for 30 min at 43 °C [58][16]. This protocol was also used in a prospective multicenter trial, PRODIGE-7. Patients (n = 265) with stage IV colorectal cancer with isolated peritoneal metastases and a PCI < 25 were randomly assigned to CRS or CRS with an oxaliplatin-based HIPEC for 30 min. No significant benefit regarding median overall survival could be shown by the addition of HIPEC (median OS 41.7 versus 41.2 months). Despite this result, the subgroup of patients with a PCI > 11 ≤ 15 demonstrated a significantly higher overall survival after CRS/HIPEC compared with CRS only [59][17]. The efficacy of HIPEC in general, with other drug combination, or at other concentrations cannot be answered by this study. Based on the results from both treatment groups, the main message of PRODIGE7 is that CRS, performed in expert centers, provides superior outcomes in patients with PM from CRC. The optimal regimen for HIPEC and the added benefit remains elusive [59][17].
2. The Rapid Development of Systemic Chemotherapy
| PM—CRC Outcome after Different Treatment Approaches | ||||||
|---|---|---|---|---|---|---|
| mOS (Months) | ||||||
| CRS/HIPEC | Systemic Chemotherapy | PIPAC | Amount of Disease | Used Drug | ||
| Vervaal V. et al., 2003 [60] | Vervaal V. et al., 2003 [31] | 22.2 | 12.6 | - | +++ | 5FU |
| Elias D. et al., 2009 [58] | Elias D. et al., 2009 [16] | 67.7 | 23.9 | - | ++ | FOLFOX/FOLFIRI |
| Franko J. et al., 2016 [5] | Franko J. et al., 2016 [29] | 16.3 | - | - | NA | FOLFOX/FOLFIRI +/−ab |
| Cremolini Ch. et al., 2020 [75] | Cremolini Ch. et al., 2020 [32] | - | 28.9 | - | NA | FOLFOXIRI |
| Quenet F. et al., 2021 [59] | Quenet F. et al., 2021 [17] | 41 | - | - | ++ | FOLFOX/FOLFIRI |
| Breuer E. et al., 2021 [4] | Breuer E. et al., 2021 [28] | 51 | - | - | ++ | FOLFOX/FOLFIRI |
| Demtröder C. et al., 2015 [76] | Demtröder C. et al., 2015 [33] | - | - | 15.7 | +++ | FOLFOX/FOLFIRI +/−ab |
| Goére D. et al., 2020 [77] | Goére D. et al., 2020 [34] | mOS not reached during 50.8 months of follow-up | - | + | FOLFOX/XELOX | |
3. Novel Concepts and New Treatment Strategies for the Treatment of PM
Apart from using HIPEC as a complementary treatment, directly after cytoreductive surgery, some novel concepts have been introduced. Many of them highlight the palliative aspect of PM. A good example is pressurized intraperitoneal aerosol chemotherapy (PIPAC), where low-dose chemotherapy is applied intraperitoneally during laparoscopy as an aerosol at a pressure of 12 mmHg, usually every four weeks. This method is currently used in patients with non-resectable disease and is well tolerated [78][35]. The rationale for PIPAC is a more homogeneous intraperitoneal distribution of chemotherapy in a closed space by applying it as a pressurized aerosol, leading to increased chemotherapy concentrations within the tissue compared with HIPEC [77,78][34][35]. PIPAC also offers a possibility to repeat the treatment. A further development of PIPAC is electrostatic PIPAC (ePIPAC). Here, a phase II study (NCT03246321) has completed patient recruitment using ePIPAC with oxaliplatin as a palliative monotherapy for patients with isolated and unresectable PM-CRC. There are multiple challenges in the diagnosis and treatment of peritoneal metastasis. A major issue is the detection of minimal disease during surgery, where scars from previous procedures are impossible to differentiate from metastatic lesions. Techniques such as fluorescent-guided surgery could amplify detection sensitivity, making resections more precise. Several therapeutic targets have being tested; among them, anti-CEA antibodies have shown encouraging clinical results [79][36]. Another field of research is the delivery of drugs into the peritoneal cavity. Here, apart from HIPEC or PIPAC, several technologies have been investigated that may improve the exposure of PM lesions to cytostatic drugs. For example, nanoparticles could provide several advantages, e.g., prolonged drug retention time, controlled drug release, and serve as a versatile vector for different molecules [80][37]. The combined used of microspheres and hyaluronic acid hydrogel showed good results, enhanced drug solubility, prolonged drug release and sustained biocompatibility [81][38]. Another innovative concept is NIPS, where a intraperitoneal catheter is placed into the pelvic cavity to administer neoadjuvant intraperitoneal and systemic chemotherapy. In patients with peritoneal metastasis from gastric cancer, NIPS resulted in a lower cancer-cell-positive ascites rate and in a higher R0 resection rate [82][39]. Given the increasing knowledge on anti-tumor immunity in the peritoneum, immune checkpoint inhibitors may play a role, not only in micro-satellite instability-high (MSI-H), or mismatch repair-deficient (MMRd) metastatic colorectal or ovarian cancer [83,84,85][40][41][42]. Although the immune cell landscape in PM lesions is not yet established, locoregional treatment may influence immunity in PM lesions. For example, anthracyclines—such as doxycycline—induce immunogenic cell death (ICD) [86][43], which leads to the release of DAMPs from dying cancer cells. DAMPs in combination with antigen release can result in DC maturation and antigen presentation to CD8+ T cells [86,87][43][44]. After recognizing a specific antigen, T cells clonally expand and can fight surviving cancer cells. Moreover, cisplatin (also used for HIPEC) sensitized spontaneous lung cancer in a mouse model to induce a checkpoint blockade via the induction of ICD. This immunogenic effect is mediated not only by chemotherapy, but also radiotherapy; a single carefully selected dose is able to mediate similar effects. Sharma et al. described an enhanced expression of cancer testis antigens and MHC-I expression after the application of 20 Gy radiotherapy in vitro [88][45]. Taken together, locoregional therapy may induce ICD to activate the immune system. In the context of HIPEC, the addition of hyperthermia might have an additional effect through the induction of heat shock proteins. In mice, an Hsp-90-mediated anticancer immune response was observed after the in vitro HIPEC treatment of murine cancer cells [89][46]. An interesting example for a novel treatment approach for PM-CRC was provided by the phase 1 ImmunoPeCa trial. The immunotoxin MOC31PE was intraperitoneally administered one day after CRS and HIPEC, with the intention of killing EpCAM-positive cancer cells and preventing recurrence [90][47]. Despite the recent progress made, a deeper insight and molecular understanding of effects during locoregional treatment on the human host physiology and the immune system is required. Investigating tumor samples from PM patients and further assessments of cytokine profiles before and after treatment could provide critical knowledge about these processes. If surgery and locoregional treatment can induce anti-tumor immunity, these patients might profit from an additional treatment to boost the immune reaction. The rationale behind such a novel treatment approach is shown in Figure 1.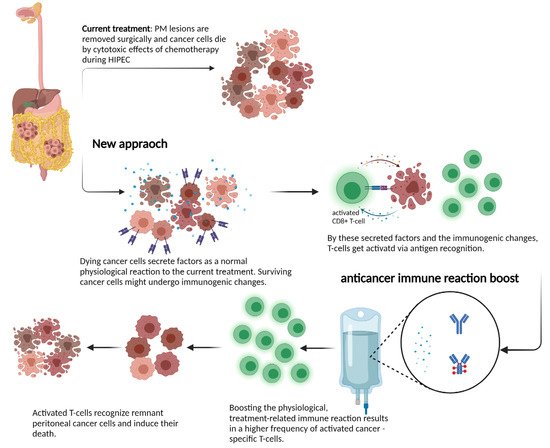
References
- Sugarbaker, P.H. Surgical management of carcinomatosis from colorectal cancer. Clin. Colon Rectal Surg. 2005, 18, 190–203.
- Neuwirth, M.G.; Alexander, H.R.; Karakousis, G.C. Then and now: Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J. Gastrointest. Oncol. 2016, 7, 18–28.
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550.
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010, 116, 3756–3762.
- Munnell, E.W. The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarian carcinoma 1952-1961. Am. J. Obstet. Gynecol. 1968, 100, 790–805.
- Munnell, E.W. Surgical treatment of ovarian carcinoma. Clin. Obstet. Gynecol. 1969, 12, 980–992.
- Griffiths, C.T.; Parker, L.M.; Fuller, A.F. Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat Rep. 1979, 63, 235–240.
- Long, R.T.; Spratt, J.S.; Dowling, E. Pseudomyxoma peritonei. New concepts in management with a report of seventeen patients. Am. J. Surg. 1969, 117, 162–169.
- Dedrick, R.L.; Myers, C.E.; Bungay, P.M.; DeVita, V.T. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978, 62, 1–11.
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42.
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996, 82, 359–374.
- Steffen, T.; Putora, P.M.; Hübner, M.; Gloor, B.; Lehmann, K.; Kettelhack, C.; Adamina, M.; Peterli, R.; Schmidt, J.; Ris, F.; et al. Diagnostic Nodes of Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Among Colorectal Cancer Patients: A Swiss National Multicenter Survey. Clin. Colorectal. Cancer 2019, 18, e335–e342.
- Glehen, O.; Mohamed, F.; Gilly, F.N. Peritoneal carcinomatosis from digestive tract cancer: New management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004, 5, 219–228.
- Van’t Sant, I.; Engbersen, M.P.; Bhairosing, P.A.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; van Driel, W.J.; Aalbers, A.G.J.; Kok, N.F.M.; Lahaye, M.J. Diagnostic performance of imaging for the detection of peritoneal metastases: A meta-analysis. Eur. Radiol. 2020, 30, 3101–3112.
- Passot, G.; Dumont, F.; Goéré, D.; Arvieux, C.; Rousset, P.; Regimbeau, J.M.; Elias, D.; Villeneuve, L.; Glehen, O.; Group, B.-R.S.W. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br. J. Surg. 2018, 105, 663–667.
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685.
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266.
- Schneider, M.A.; Eshmuminov, D.; Lehmann, K. Major Postoperative Complications Are a Risk Factor for Impaired Survival after CRS/HIPEC. Ann. Surg. Oncol. 2017, 24, 2224–2232.
- Grass, F.; Vuagniaux, A.; Teixeira-Farinha, H.; Lehmann, K.; Demartines, N.; Hübner, M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br. J. Surg. 2017, 104, 669–678.
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2015, 22, 2656–2662.
- Virzi, S.; Iusco, D.; Baratti, D.; Bonomi, S.; Grassi, A.; Kusamura, S.; Deraco, M. Pilot study of adjuvant hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer at high risk for the development of peritoneal metastases. Tumori 2013, 99, 589–595.
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770.
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.J.; Tveit, K.M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal. Cancer 2015, 14, 1–10.
- Hayashi, K.; Jiang, P.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007, 67, 8223–8228.
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618.
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676.
- Saito, S.; Okabe, H.; Watanabe, M.; Ishimoto, T.; Iwatsuki, M.; Baba, Y.; Tanaka, Y.; Kurashige, J.; Miyamoto, Y.; Baba, H. CD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancer. Oncol. Rep. 2013, 29, 1570–1578.
- Breuer, E.; Hebeisen, M.; Schneider, M.A.; Roth, L.; Pauli, C.; Frischer-Ordu, K.; Eden, J.; Pache, B.; Steffen, T.; Hubner, M.; et al. Site of Recurrence and Survival after Surgery for Colorectal Peritoneal Metastasis. J. Natl. Cancer. Inst. 2021.
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet. Oncol. 2016, 17, 1709–1719.
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507.
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743.
- Passot, G.; Vaudoyer, D.; Cotte, E.; You, B.; Isaac, S.; Noel Gilly, F.; Mohamed, F.; Glehen, O. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann. Surg. 2012, 256, 125–129.
- Demtroder, C.; Solass, W.; Zieren, J.; Strumberg, D.; Giger-Pabst, U.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal. Dis. 2016, 18, 364–371.
- Goere, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154.
- Solass, W.; Kerb, R.; Mürdter, T.; Giger-Pabst, U.; Strumberg, D.; Tempfer, C.; Zieren, J.; Schwab, M.; Reymond, M.A. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann. Surg. Oncol. 2014, 21, 553–559.
- Hollandsworth, H.M.; Turner, M.A.; Hoffman, R.M.; Bouvet, M. A review of tumor-specific fluorescence-guided surgery for colorectal cancer. Surg. Oncol. 2021, 36, 84–90.
- Shariati, M.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Aerosolization of Nanotherapeutics as a Newly Emerging Treatment Regimen for Peritoneal Carcinomatosis. Cancers 2019, 11, 906.
- Luo, J.; Wu, Z.; Lu, Y.; Xiong, K.; Wen, Q.; Zhao, L.; Wang, B.; Gui, Y.; Fu, S. Intraperitoneal administration of biocompatible hyaluronic acid hydrogel containing multi-chemotherapeutic agents for treatment of colorectal peritoneal carcinomatosis. Int J. Biol. Macromol. 2020, 152, 718–726.
- Zhang, X.; Huang, H.; Yang, D.; Wang, P.; Huang, X.; Hu, Z.; Zhang, Y.; Yan, R.; Zhu, Z.; Cai, Q. Neoadjuvant Intraperitoneal and Systemic Chemotherapy Versus Neoadjuvant Systemic Chemotherapy with Docetaxel, Oxaliplatin, and S-1 for Gastric Cancer with Peritoneal Metastasis: A Propensity Score Matched Analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211036310.
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779.
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218.
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087.
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010, 29, 482–491.
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363.
- Sharma, A.; Bode, B.; Wenger, R.H.; Lehmann, K.; Sartori, A.A.; Moch, H.; Knuth, A.; Boehmer, L.; Broek, M. gamma-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS ONE 2011, 6, e28217.
- Zunino, B.; Rubio-Patino, C.; Villa, E.; Meynet, O.; Proics, E.; Cornille, A.; Pommier, S.; Mondragon, L.; Chiche, J.; Bereder, J.M.; et al. Hyperthermic intraperitoneal chemotherapy leads to an anticancer immune response via exposure of cell surface heat shock protein 90. Oncogene 2016, 35, 261–268.
- Froysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Oien, J.T.; Olsen, K.H.; Giercksky, K.E.; Julsrud, L.; Fodstad, O.; Dueland, S.; et al. Novel Treatment with Intraperitoneal MOC31PE Immunotoxin in Colorectal Peritoneal Metastasis: Results From the ImmunoPeCa Phase 1 Trial. Ann. Surg. Oncol. 2017, 24, 1916–1922.
