The identification of viral RNA using reverse transcription quantitative polymerase chain reaction (RT-qPCR) is the gold standard for identifying an infection caused by SARS-CoV-2. The limitations of RT-qPCR such as requirement of expensive instruments, trained staff and laboratory facilities led to development of rapid antigen tests (RATs). The performance of RATs has been widely evaluated and found to be varied in different settings. The present systematic review aims to evaluate the pooled sensitivity and specificity of the commercially available RATs. The overall pooled sensitivity and specificity of RATs were 70% (95% CI: 69–71) and 98% (95% CI: 98–98), respectively. In subgroup analyses, nasal swabs showed the highest sensitivity of 83% (95% CI: 80–86) followed by nasopharyngeal swabs 71% (95% CI: 70–72), throat swabs 69% (95% CI: 63–75) and saliva 68% (95% CI: 59–77). Samples from symptomatic patients showed a higher sensitivity of 82% (95% CI: 82–82) as compared to asymptomatic patients at 68% (95% CI: 65–71), while a cycle threshold (Ct) value ≤25 showed a higher sensitivity of 96% (95% CI: 95–97) as compared to higher Ct value. Although the sensitivity of RATs needs to be enhanced, it may still be a viable option in places where laboratory facilities are lacking for diagnostic purposes in the early phase of disease.
- SARS-CoV-2
- rapid antigen test
- sensitivity
- specificity
1. Introduction
2. Performance of Rapid Antigen Tests for COVID-19 Diagnosis
Most of the studies still from European countries such as Germany, Spain, Italy, the Netherlands, France, Belgium and Switzerland. Lack of studies from West and Southeast Asian countries, South America and Africa, highlighting the current gap pertaining to understanding the RATs performance in such geographical areas. A similar observation was reported by the previous study, in which, most of the included studies were from Germany, Spain and Italy [17][7]. The plausible reason may be attributed to the fact that most kits are manufactured in European countries; thus, such test kits were easier to obtain in those countries as compared to others where supply shortages are commonly reported. The second reason is that these countries were badly affected by COVID-19 in the beginning of the outbreak. Therefore, the COVID-19 RAT is becoming popular across European countries as governments’ efforts to slow the spreading of the virus by tracking infected individuals.
Panbio™ COVID-19 Ag RDT (Abbott, Jena, Germany) and STANDARD Q COVID-19 Ag Home Test (SD Biosensor, Seoul, South Korea) were used in the majority of studies (25 and 11 studies, respectively) followed by SARS-CoV-2 Rapid Ag Test (Roche, Basel, Switzerland) and Lumipulse G SARS-CoV-2 Ag (Fujirebio, Tokyo, Japan). This could be due to the fact that Panbio™ COVID-19 Ag RDT (Abbott, Jena, Germany) and STANDARD Q COVID-19 Ag Home Test (SD Biosensor, Seoul, South Korea) are two RAD kits that are currently included under the ‘WHO Emergency Use Listing for In vitro diagnostics (IVDs) Detecting SARS-CoV-2′ [113][8]. There is still a lack of evaluation for newly developed test kits such as CoviNAg ELISA Kit (XEMA, Moscow, Russia) and Sienna-Clarity COVID-19 Ag RTC (Salofa Oy, Salo, Finland). Therefore, future diagnostic evaluation studies should include the newly developed test kits so that more data can be obtained for future comparative analyses.
Immunochromatography, which involves spotting antibodies onto nitrocellulose membranes that interact with specific antigens in patient samples is the basis of RATs. The antigen–antibody interaction can be visualised manually or by using an immunofluorescence machine reader. The genome of SARS-CoV-2 comprises genes the responsible for four structural proteins such the spike (S), envelope (E), membrane (M) and nucleocapsid (N) [114][9]. N protein is frequently employed as a target analyte in RATs for COVID-19 diagnosis. N-protein is mostly expressed during the early stages of SARS-CoV-2 infection and has the least amount of variation in its gene sequence, indicating that it is a stable protein [115][10].
Most studies used nasopharyngeal swabs as specimens examined for evaluating the performance of the test kits. Only a few studies used nasal swabs, saliva and throat swabs. This observation signaling the need for more studies using such specimens so that alternative sampling approaches for the rapid detection of SARS-CoV-2 could be identified. Subgroup analysis of the test kits based on specimens showed nasal swabs gave the highest sensitivity of 83% (95% CI: 80–86) followed by nasopharyngeal swabs 71% (95% CI: 70–72), throat swabs 69% (95% CI: 63–75) and saliva 68% (95% CI: 59–77). Interestingly, nasopharyngeal swabs are currently considered as the gold standard specimen for SARS-CoV-2 laboratory diagnosis. This finding may suggest that nasal swab could replace nasopharyngeal swabs as the gold standard specimen. However, it is important to note that the average sensitivity of the nasal swabs was calculated only based on 2148 samples as compared to 38548 samples for nasopharyngeal swabs. Thus, evaluation using more samples are still needed to provide a comprehensive conclusion regarding the nasal swabs’ performance. Lower sensitivity for throat swabs was in agreement with a recent study on the performances of the different sampling approaches for SARS-CoV-2, which reported lower sensitivity by throat swabs (68%) as compared to nasal swabs and saliva [116][11].
The sensitivity and specificity of the 30 RATs ranged from 37% to 90% and 65% to 100%, respectively, whereas the overall pooled sensitivity and specificity of RATs were 70% (95% CI: 69–71) and 98% (95% CI: 98–98), respectively (Figure 1). Based on sample size, the SARS-CoV-2 Ag Test (LumiraDx GmbH, Cologne, Germany) showed the highest sensitivity of 86% followed by the Lumipulse G SARS-CoV-2 Ag (Fujirebio, Tokyo, Japan) and BinaxNOW™ COVID-19 Ag Self-Test (Abbott, Jena, Germany) with sensitivities of 83% and 79%, respectively. The different results could be related to various study methods, RAT kits manufacturers, patient selection, types of specimens and the stage of disease at the time of sample collection. Based on the meta-analysis, the RAT kits that meet WHO criteria are Sienna-Clarity COVID-19 Ag RTC (Salofa Oy, Salo, Finland), Biosynex COVID-19 Ag BSS Test (Biosynex, Strasbourg, France), Innova Rapid SARS-CoV-2 Ag Test (Xiamen Biotime Biotechnology, Fujian, China), Dräger Ag Test SARS-CoV-2 (Dräger, Lübeck, Germany), SARS-CoV-2 Ag Test (LumiraDx GmbH, Cologne, Germany), Lumipulse G SARS-CoV-2 Ag (Fujirebio, Tokyo, Japan), S-PLEX SARS-CoV-2 N Kit (MesoScale Diagnostics, Maryland, USA) and BinaxNOW™ COVID-19 Ag Self-Test (Abbott, Jena, Germany).
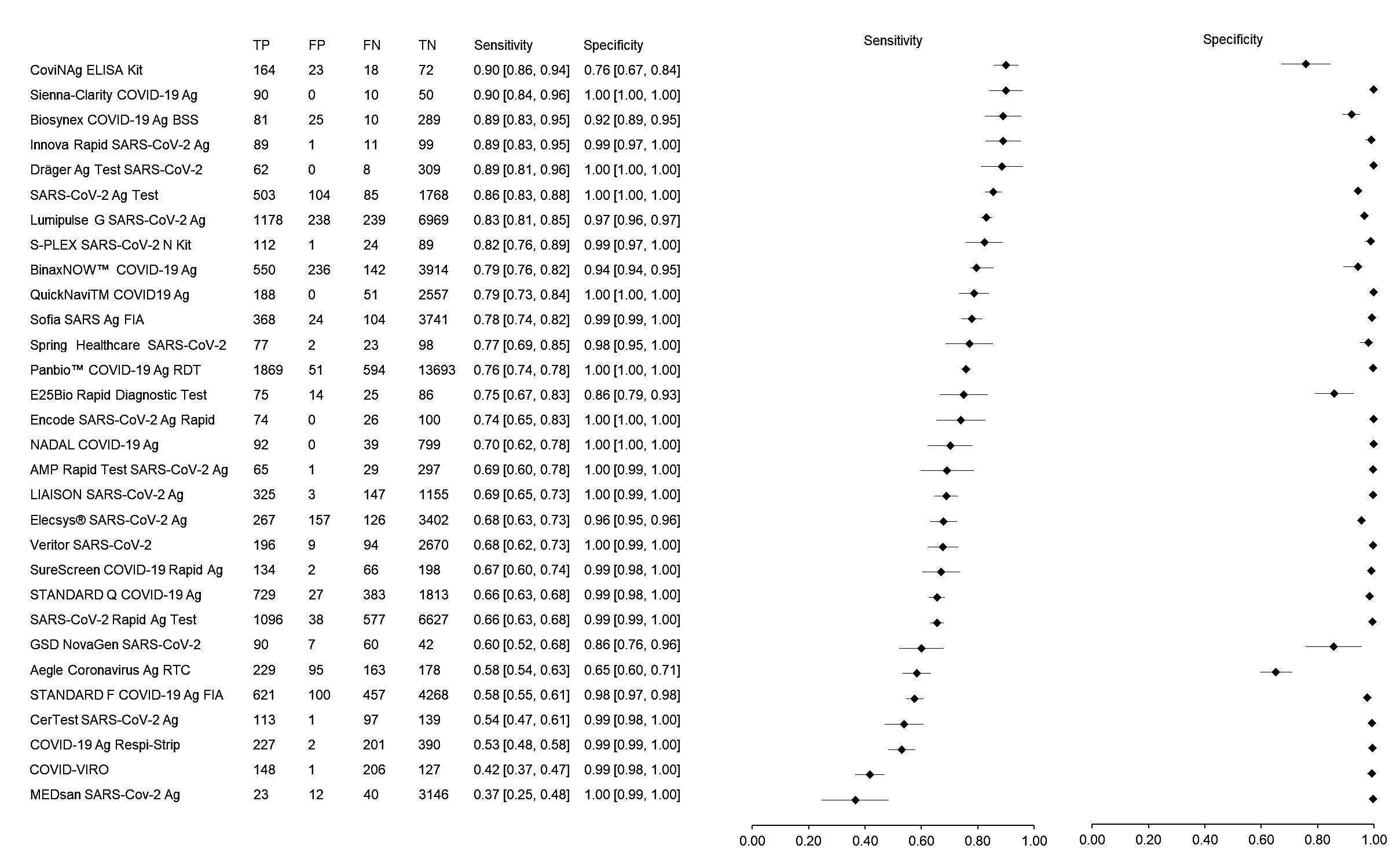
Figure 1.
The forest plot of sensitivity and specificity of rapid antigen tests for SARS-CoV-2
According to the WHO, RATs should be prioritized for use in symptomatic individuals who meet the COVID-19 case definition, as well as to test asymptomatic individuals at high risk of infection, particularly in settings where NAAT testing capacity is limited. RATs showed similar specificity (98%) for symptomatic and asymptomatic patients. However, the sensitivity of RATs for symptomatic patients was greater than asymptomatic patients (82% vs. 68%). A similar finding was reported by the previous systematic reviewudies, in which, the sensitivity of RATs is higher when used for symptomatic patients [16,17][12][7]. In addition, there is a clear association between Ct values of RT-qPCR and RATs’ sensitivity and specificity. The lower the Ct value (≤25), the greater the sensitivity and specificity of RATs, whereas the higher the Ct value (<25), the lower the sensitivity and specificity of RATs. Ct values, on the other hand, cannot be directly compared between tests and must be interpreted with caution because they are impacted by sample type, sample collection timing, and assay design [118][13].
The severity of the disease, the timing of sample collection, the types of samples, and sample handling techniques all influence antigen levels in samples [20][14]. It is hard to determine if the difference in observed sensitivity is due to the test’s performance or the qualities of the samples utilized in the test without this information. Unfortunately, the majority of the studies did not provide information on antigen levels in the samples. Information about disease severity and sample collection timing are often missing. Future research should include this information to enable for a more accurate assessment of diagnosis test performance as well as the identification of their actual limitations.
53. Conclusions
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Saniasiaya, J.; Islam, M.A.; Abdullah, B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. Laryngoscope 2021, 131, 865–878.
- Saniasiaya, J.; Islam, M.A.; Abdullah, B. Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-Analysis of 29,349 Patients. Otolaryngol.—Head Neck Surg. 2021, 165, 33–42.
- Subali, A.D.; Wiyono, L. Reverse Transcriptase Loop Mediated Isothermal AmplifiCation (RT-LAMP) for COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. Pathog. Glob. Health 2021, 115, 281–291.
- Lu, X.; Wang, L.; Sakthivel, S.K.; Whitaker, B.; Murray, J.; Kamili, S.; Lynch, B.; Malapati, L.; Burke, S.A.; Harcourt, J.; et al. US CDC Real-Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1654–1665.
- Shen, M.; Zhou, Y.; Ye, J.; Abdullah AL-maskri, A.A.; Kang, Y.; Zeng, S.; Cai, S. Recent Advances and Perspectives of Nucleic Acid Detection for Coronavirus. J. Pharm. Anal. 2020, 10, 97–101.
- Barbić, L.; Savić, V.; Bogdanić, M.; Antolašić, L.; Milašinčić, L.; Hruškar, Ž.; Sabadi, D.; Perić, L.; Tabain, I.; Stevanović, V.; et al. Diagnosis of SARS-CoV-2 Infection. Infektološki Glas 2020, 40, 50–54.
- Khandker, S.S.; Nik Hashim, N.H.H.; Deris, Z.Z.; Shueb, R.H.; Islam, M.A. Diagnostic Accuracy of Rapid Antigen Test Kits for Detecting SARS-CoV-2: A Systematic Review and Meta-Analysis of 17,171 Suspected COVID-19 Patients. J. Clin. Med. 2021, 10, 3493.
- Mak, G.C.K.; Lau, S.S.Y.; Wong, K.K.Y.; Chow, N.L.S.; Lau, C.S.; Lam, E.T.K.; Chan, R.C.W.; Tsang, D.N.C. Evaluation of Rapid Antigen Detection Kit from the WHO Emergency Use List for Detecting SARS-CoV-2. J. Clin. Virol. 2021, 134, 104712.
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e10.
- Surjit, M.; Lal, S.K. The SARS-CoV Nucleocapsid Protein: A Protein with Multifarious Activities. Infect. Genet. Evol. 2008, 8, 397–405.
- Tsang, N.N.Y.; So, H.C.; Ng, K.Y.; Cowling, B.J.; Leung, G.M.; Ip, D.K.M. Diagnostic Performance of Different Sampling Approaches for SARS-CoV-2 RT-PCR Testing: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2021, 21, 1233–1245.
- Brümmer, L.E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; Pollock, N.R.; et al. Accuracy of Novel Antigen Rapid Diagnostics for SARS-CoV-2: A Living Systematic Review and Meta-Analysis. PLoS Med. 2021, 18, e1003735.
- Shah, V.P.; Farah, W.H.; Hill, J.C.; Hassett, L.C.; Binnicker, M.J.; Yao, J.D.; Murad, M.H. Association Between SARS-CoV-2 Cycle Threshold Values and Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2021, 8, ofab453.
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 Antigen Detection Assay in Comparison with Real-Time RT-PCR Assay for Laboratory Diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177.
