Knotted-like homeobox (KNOX) genes encode homeodomain-containing transcription factors (TFs) that modulate various important developmental processes in plants. While Class I KNOX TF genes are mainly expressed in the shoot apical meristems of both monocot and eudicot plants and are involved in meristem maintenance and/or formation, Class II KNOXTF genes exhibit diverse expression patterns and their precise functions have mostly remained unknown. The expression patterns of Class II KNOX TF genes in Arabidopsis, namely KNAT3, KNAT4, KNAT5, and KNAT7, suggest that TFs encoded by at least some of these genes, such as KNAT7 and KNAT3, may play a significant role in secondary cell wall formation.
- bioethanol
- KNOX II transcription factors
- saccharification
- secondary cell walls
- xylan
- xylem and fiber development
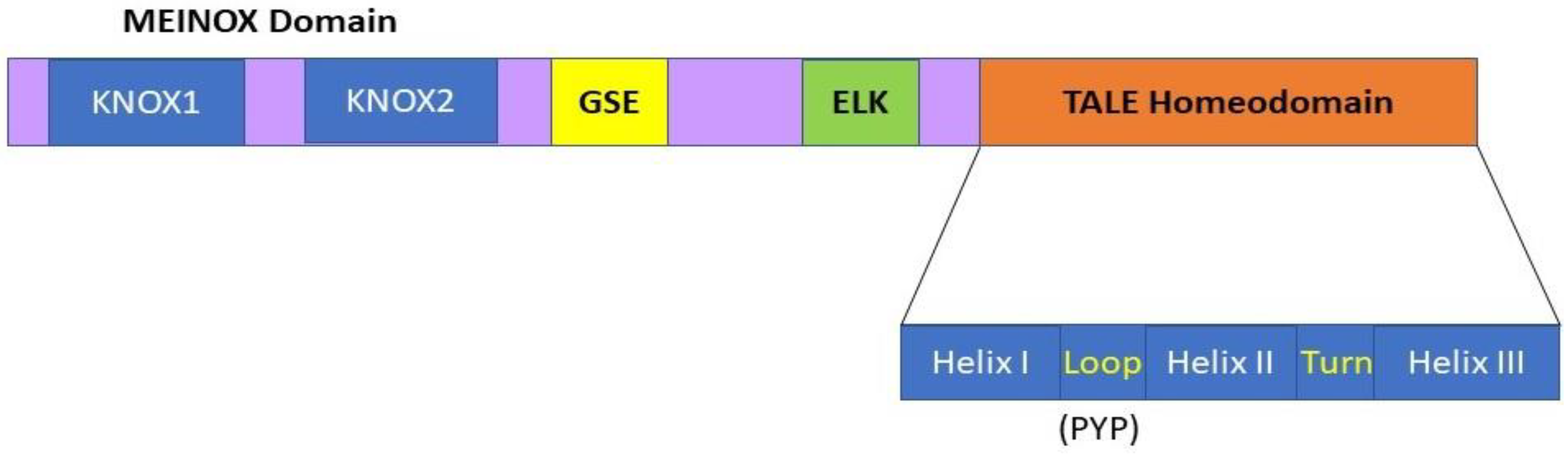
1. KNOX Genes and Encoded KNOX Proteins in Plants
2. The Expression Patterns of Class II KNOX Genes in Plants Provide Some Clues about Their Functionality in SCW Formation
3. Genetic Mutations in Class II KNOX Genes Further Clarify Their Role in SCW Formation
| Target Gene | Mutation | Type of Mutation | Anatomy of Mutants | References | |||
|---|---|---|---|---|---|---|---|
| AtKNAT7 | irx11 | T-DNA insertion | Irregular xylem with collapsed vessels. | [18] | |||
| AtKNAT7 | - | Dominant repression | |||||
| Cotton GhKNL1 | Arabidopsis | Overexpression | Reduced cell wall thickness of both xylem vessels and fibers; reduced composition of several monosaccharides from the cell walls. | [13] | |||
| Thinner interfascicular fibers and slightly thinner vessel walls, but no change in xylary fibers. | [ | 22 | ] | AtKNAT7 | irx11 | Loss-of-function mutation | Thinner vessels walls resulted in a collapse of xylem vessels that showed the |
| Cotton GhKNAT7 | Arabidopsis | Overexpression | irx phenotype and thicker interfascicular fibers compared to controls; increase in lignin content. | [14 | Reduced deposition of lignocellulose in interfascicular fibers, but no change in the SCWs of xylem fibers and vessels.] | ||
| [ | 7 | ] | AtKNAT3, AtKNAT4, AtKNAT5 | Single mutants | T-DNA insertion | ||
| NbKNAT7 | Tobacco | Downregulation by VIGS and RNAi | No irx phenotype. | Increased xylem proliferation with thin-walled fiber cells, increased polysaccharide extractability, and higher saccharification rate.[16] | |||
| [ | 15 | ] | KNAT3/KNAT7 | Double mutant | T-DNA insertion | Enhanced irregular xylem ( | |
| AtKNAT7 | Arabidopsis | Dominant repression | irx) phenotype characterized by weak inflorescence stem; reduced interfascicular fiber wall thickness and modified cell wall composition. | Reduced expression of SCW genes that resulted in thinner fiber cell walls with altered cell wall composition.[16] | |||
| [ | 13 | KNAT3/KNAT7 | Double mutant | Chimeric repression | Thinner interfascicular fiber cell walls compared to single mutants and wild type (WT); reduced cellulose and xylan and reduced S/G lignin ratio. | [17] | |
| OsKNAT7 | CRISPR/CAS9 | T-DNA insertion | Thicker fiber cell walls; larger grain size due to cell expansion in spikelet bracts. | [24] | |||
| GhKNL1 | - | Dominant repression | Abnormal shorter fiber length. | [22] |
4. Targeted Genetic Manipulations in Class II KNOX Genes Confirm Their Role in SCW Formation
| Gene Used | Target Plant | Gene Modification Method | Impact on Transgenic Plants | References |
|---|---|---|---|---|
| AtKNAT7 | Arabidopsis | Overexpression | Thin interfascicular fiber walls, but no change in vessel wall thickness. | [14] |
| ] | ||||
| PtKNAT7 | ||||
| Poplar | Overexpression | Enhanced expression of SCW genes, CesA8, IRX9, PAL, and CCR. | [ | 25] |
| PtKNAT7 | Poplar | Downregulation by antisense | Reduced expression of SCW genes, reduced lignin content, altered lignin composition (S/G ratio), and increased saccharification. | [25] |
5. Transcriptional Network of the Class II KNOX Genes Involved in SCW Formation
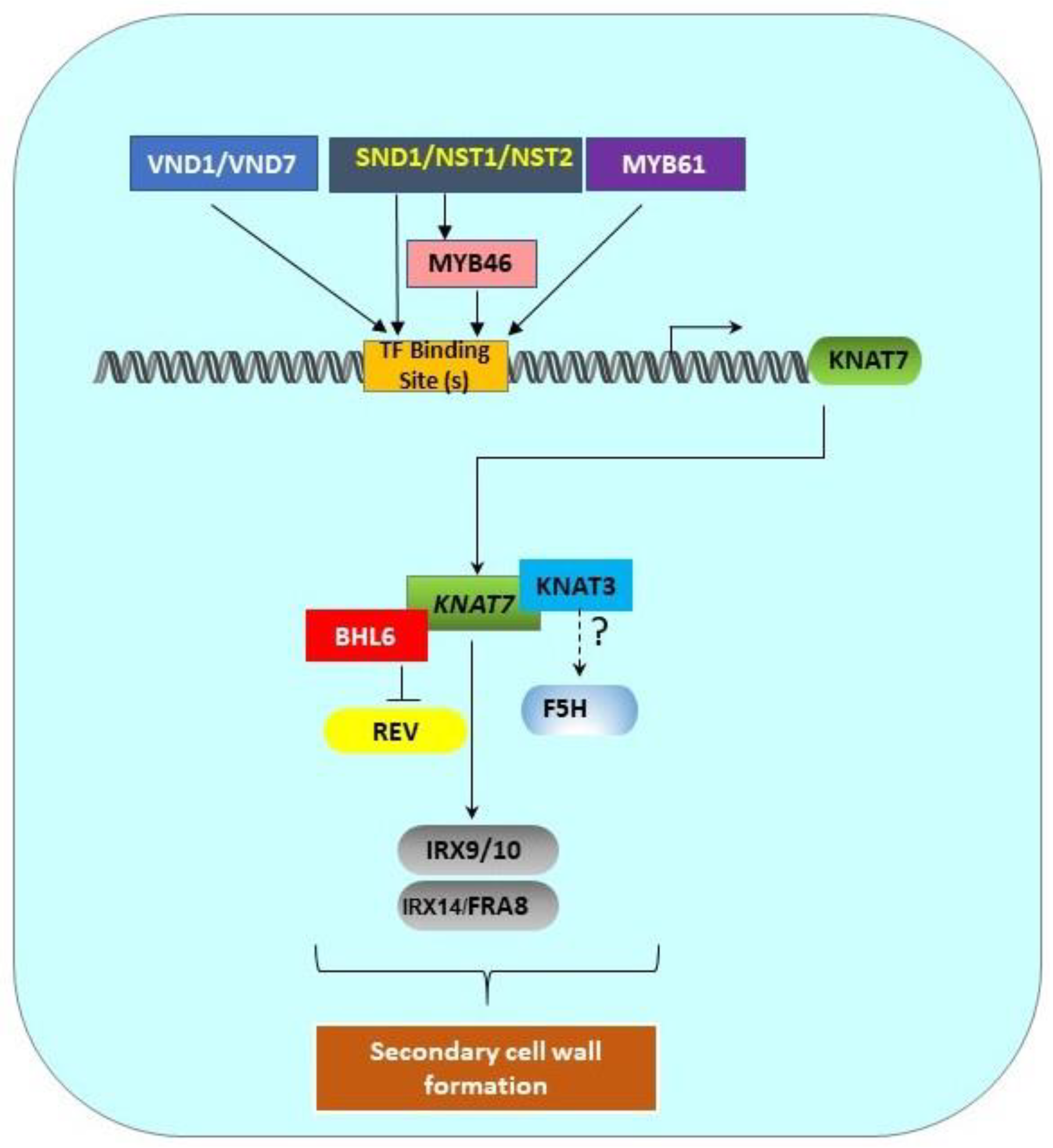
Figure 2. Transcriptional regulation pathway of KNAT7 gene. SCW-associated upstream transcription factors (MYB61, SND1/NST1/NST2, VND1/VND7) and MYB46 directly bind the binding sites in the KNAT7 gene promoter to regulate the expression of the KNAT7 gene. KNAT7 positively regulates the expression of various xylan synthesis genes (IRX9/10 and IRX14L/FRA8). Interactions between KNAT7 and KNAT3 TFs might regulate F5H expression, and the interactions between KNAT7 and BLH6 negatively regulate the expression of the homeodomain-ZIP (HD-ZIP) TF gene Revoluta. All these interactions ultimately regulate SCW formation in higher plants. All genes are shown as rounded rectangles and proteins are indicated by rectangles.
References
- Sakakibara, K.; Ando, S.; Yip, H.K.; Tamada, Y.; Hiwatashi, Y.; Murata, T.; Deguchi, H.; Hasebe, M.; Bowman, J.L. KNOX2 Genes Regulate the Haploid-to-Diploid Morphological Transition in Land Plants. Science 2013, 339, 1067–1070.
- Desplan, C.; Theis, J.; O’Farrell, P.H. The Sequence Specificity of Homeodomain-DNA Interaction. Cell 1988, 54, 1081–1090.
- Burglin, T.R. Analysis of TALE Superclass Homeobox Genes (MEIS, PBC, KNOX, Iroquois, TGIF) Reveals a Novel Domain Conserved between Plants and Animals. Nucleic Acids Res. 1997, 25, 4173–4180.
- Kerstetter, R.; Vollbrecht, E.; Lowe, B.; Veit, B.; Yamaguchi, J.; Hake, S. Sequence Analysis and Expression Patterns Divide the Maize Knotted1-like Homeobox Genes into Two Classes. Plant Cell 1994, 6, 1877–1887.
- Bharathan, G.; Janssen, B.J.; Kellogg, E.A.; Sinha, N. Phylogenetic Relationships and Evolution of the KNOTTED Class of Plant Homeodomain Proteins. Mol. Biol. Evol. 1999, 16, 553–563.
- Xiong, H.; Shi, A.; Wu, D.; Weng, Y.; Qin, J.; Ravelombola, W.S.; Shu, X.; Zhou, W. Genome-Wide Identification, Classification and Evolutionary Expansion of KNOX Gene Family in Rice (Oryza Sativa) and Populus (Populustrichocarpa). Am. J. Plant Sci. 2018, 9, 1071–1092.
- Ma, Q.; Wang, N.; Hao, P.; Sun, H.; Wang, C.; Ma, L.; Wang, H.; Zhang, X.; Wei, H.; Yu, S. Genome-Wide Identification and Characterization of TALE Superfamily Genes in Cotton Reveals Their Functions in Regulating Secondary Cell Wall Biosynthesis. BMC Plant Biol. 2019, 19, 432.
- Vollbrecht, E.; Veit, B.; Sinha, N.; Hake, S. The Developmental Gene Knotted-1 Is a Member of a Maize Homeobox Gene Family. Nature 1991, 350, 241–243.
- Hake, S.; Smith, H.M.S.; Holtan, H.; Magnani, E.; Mele, G.; Ramirez, J. The Role of Knox Genes in Plant Development. Annu. Rev. Cell Dev. Biol. 2004, 20, 125–151.
- Sakamoto, T.; Sato, Y.; Matsuoka, M. Function of KNOX Homeodomain Proteins in Plant Development. Plant Biotechnol. 2001, 18, 85–92.
- Kimura, S.; Koenig, D.; Kang, J.; Yoong, F.Y.; Sinha, N. Natural Variation in Leaf Morphology Results from Mutation of a Novel KNOX Gene. Curr. Biol. 2008, 18, 672–677.
- Di Giacomo, E.; Sestili, F.; Iannelli, M.A.; Testone, G.; Mariotti, D.; Frugis, G. Characterization of KNOX Genes in Medicago Truncatula. Plant Mol. Biol. 2008, 67, 135–150.
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.-H. A Battery of Transcription Factors Involved in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782.
- Li, E.; Bhargava, A.; Qiang, W.; Friedmann, M.C.; Forneris, N.; Savidge, R.A.; Johnson, L.A.; Mansfield, S.D.; Ellis, B.E.; Douglas, C.J. The Class II KNOX Gene KNAT7 Negatively Regulates Secondary Wall Formation in Arabidopsis and Is Functionally Conserved in Populus. New Phytol. 2012, 194, 102–115.
- Pandey, S.K.; Nookaraju, A.; Fujino, T.; Pattathil, S.; Joshi, C.P. Virus-Induced Gene Silencing (VIGS)-Mediated Functional Characterization of Two Genes Involved in Lignocellulosic Secondary Cell Wall Formation. Plant Cell Rep. 2016, 35, 2353–2367.
- Wang, S.; Yamaguchi, M.; Grienenberger, E.; Martone, P.T.; Samuels, A.L.; Mansfield, S.D. The Class II KNOX Genes KNAT3 and KNAT7 Work Cooperatively to Influence Deposition of Secondary Cell Walls That Provide Mechanical Support to Arabidopsis Stems. Plant J. 2020, 101, 293–309.
- Qin, W.; Yin, Q.; Chen, J.; Zhao, X.; Yue, F.; He, J.; Yang, L.; Liu, L.; Zeng, Q.; Lu, F.; et al. The Class II KNOX Transcription Factors KNAT3 and KNAT7 Synergistically Regulate Monolignol Biosynthesis in Arabidopsis. J. Exp. Bot. 2020, 71, 5469–5483.
- Brown, D.M.; Zeef, L.A.H.; Ellis, J.; Goodacre, R.; Turner, S.R. Identification of Novel Genes in Arabidopsis Involved in Secondary Cell Wall Formation Using Expression Profiling and Reverse Genetics. Plant Cell 2005, 17, 2281–2295.
- He, J.-B.; Zhao, X.-H.; Du, P.-Z.; Zeng, W.; Beahan, C.T.; Wang, Y.-Q.; Li, H.-L.; Bacic, A.; Wu, A.-M. KNAT7 Positively Regulates Xylan Biosynthesis by Directly Activating IRX9 Expression in Arabidopsis: KNAT7 Positively Regulates Xylan Biosynthesis. J. Integr. Plant Biol. 2018, 60, 514–528.
- Ehlting, J.; Mattheus, N.; Aeschliman, D.S.; Li, E.; Hamberger, B.; Cullis, I.F.; Zhuang, J.; Kaneda, M.; Mansfield, S.D.; Samuels, L.; et al. Global Transcript Profiling of Primary Stems from Arabidopsis Thaliana Identifies Candidate Genes for Missing Links in Lignin Biosynthesis and Transcriptional Regulators of Fiber Differentiation: Global Transcript Profiling of Stems. Plant J. 2005, 42, 618–640.
- Persson, S.; Wei, H.; Milne, J.; Page, G.P.; Somerville, C.R. Identification of Genes Required for Cellulose Synthesis by Regression Analysis of Public Microarray Data Sets. Proc. Natl. Acad. Sci. USA 2005, 102, 8633–8638.
- Gong, S.-Y.; Huang, G.-Q.; Sun, X.; Qin, L.-X.; Li, Y.; Zhou, L.; Li, X.-B. Cotton KNL1, Encoding a Class II KNOX Transcription Factor, Is Involved in Regulation of Fibre Development. J. Exp. Bot. 2014, 65, 4133–4147.
- Zhong, R.; Yuan, Y.; Spiekerman, J.J.; Guley, J.T.; Egbosiuba, J.C.; Ye, Z.-H. Functional Characterization of NAC and MYB Transcription Factors Involved in Regulation of Biomass Production in Switchgrass (Panicum Virgatum). PLoS ONE 2015, 10, e0134611.
- Wang, S.; Yang, H.; Mei, J.; Liu, X.; Wen, Z.; Zhang, L.; Xu, Z.; Zhang, B.; Zhou, Y. Rice Homeobox Protein KNAT7 Integrates the Pathways Regulating Cell Expansion and Wall Stiffness. Plant Physiol. 2019, 181, 669–682.
- Ahlawat, Y.K.; Nookaraju, A.; Harman-Ware, A.E.; Doeppke, C.; Biswal, A.K.; Joshi, C.P. Genetic Modification of KNAT7 Transcription Factor Expression Enhances Saccharification and Reduces Recalcitrance of Woody Biomass in Poplars. Front. Plant Sci. 2021, 12, 762067.
- Yoo, C.G.; Dumitrache, A.; Muchero, W.; Natzke, J.; Akinosho, H.; Li, M.; Sykes, R.W.; Brown, S.D.; Davison, B.; Tuskan, G.A.; et al. Significance of Lignin S/G Ratio in Biomass Recalcitrance of Populus Trichocarpa Variants for Bioethanol Production. ACS Sustain. Chem. Eng. 2018, 6, 2162–2168.
- Ko, J.-H.; Kim, W.-C.; Han, K.-H. Ectopic Expression of MYB46 Identifies Transcriptional Regulatory Genes Involved in Secondary Wall Biosynthesis in Arabidopsis. Plant J. 2009, 60, 649–665.
- Zhong, R.; Ye, Z.-H. Complexity of the Transcriptional Network Controlling Secondary Wall Biosynthesis. Plant Sci. 2014, 229, 193–207.
- Pimrote, K. Transcriptional Regulatory Network Controlling Secondary Cell Wall Biosynthesis and Biomass Production in Vascular Plants. Afr. J. Biotechnol. 2012, 11, 13928–13937.
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.A.; Ohtani, M. NAC-MYB-Based Transcriptional Regulation of Secondary Cell Wall Biosynthesis in Land Plants. Front. Plant Sci. 2015, 6.
- Rao, X.; Dixon, R.A. Current Models for Transcriptional Regulation of Secondary Cell Wall Biosynthesis in Grasses. Front. Plant Sci. 2018, 9, 399.
- Yu, Y. OsKNAT7 Bridges Secondary Cell Wall Formation and Cell Growth Regulation. Plant Physiol. 2019, 181, 385–386.
- de Vries, L.; Guevara-Rozo, S.; Cho, M.; Liu, L.-Y.; Renneckar, S.; Mansfield, S.D. Tailoring Renewable Materials via Plant Biotechnology. Biotechnol. Biofuels 2021, 14, 167.
- Joshi, C.P.; Bhandari, S.; Ranjan, P.; Kalluri, U.C.; Liang, X.; Fujino, T.; Samuga, A. Genomics of Cellulose Biosynthesis in Poplars. New Phytol. 2004, 164, 53–61.
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546.
- Lee, C.; Teng, Q.; Zhong, R.; Ye, Z.-H. Molecular Dissection of Xylan Biosynthesis during Wood Formation in Poplar. Mol. Plant 2011, 4, 730–747.
- Polko, J.K.; Kieber, J.J. The Regulation of Cellulose Biosynthesis in Plants. Plant Cell 2019, 31, 282–296.
- Behr, M.; Guerriero, G.; Grima-Pettenati, J.; Baucher, M. A Molecular Blueprint of Lignin Repression. Trends Plant Sci. 2019, 24, 1052–1064.
- Wang, Y.; Xu, Y.; Pei, S.; Lu, M.; Kong, Y.; Zhou, G.; Hu, R. KNAT7 Regulates Xylan Biosynthesis in Arabidopsis Seed-Coat Mucilage. J. Exp. Bot. 2020, 71, 4125–4139.
