The coronavirus disease 2019 (COVID-19) pandemic, an infection caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2), has led to more than 771,000 deaths worldwide. Tobacco smoking is a major known risk factor for severe illness and even death from many respiratory infections. The effects of smoking on COVID-19 are currently controversial. Here, we provide an overview of the latest knowledge about smoking and COVID-19.
- COVID-19
- Smoking
- SARS-CoV-2
1. Introduction
Since smoking is a major risk factor for respiratory infections due to suppressive effect of the immune response, thus a hypothetical link between smoking and worsening COVID-19 can be made [1][2][25,26]. Smoking is a well-established risk factor in lung cancer, COPD, and asthma [3][27], which in turn have been shown to be risk factors for more severe illness in COVID-19 patients [4][28]. To reduce the incidence and severity of an infectious disease, understanding the impact of host factors, particularly avoidable lifestyle factors such as smoking, may play a vital role. Based on recent literature, the WHO confirmed that smokers may experience severe complications from COVID-19 than non-smokers [5][15]. Considering the complications resulting from smoking in patients with viral infections, we attempted to highlight research focusing on the association between smoking and COVID-19 outcomes, including disease severity, pathogenesis, potential molecular mechanisms, and possible therapeutic interventions.
2. The Epidemiology of Smoking and COVID-19
During the COVID-19 pandemic, there is a quest whether smoking increases the risk of acquiring respiratory infections or the biological influence of nicotine on the SAR-CoV-2 [6][7][8][29,30,31]. No strong evidence exists that support an increased risk of COVID-19 in smokers [9][32]. However, several indirect studies suggest that this population is at a higher risk for a more severe infection amongst hospitalized patients as evident by increased admission rates into ICU, use of ventilators, and death as compared to non-smokers.
Recent studies demonstrated that 1.4–18.5% of hospitalized COVID-19 adult patients were smokers [9][32]. Two meta-analyses studies (from China) were released the prevalence of COVID-19 in smoker patients. Emami et al. [10][33] identified 10 articles which covered 76,993 patients and highlighted an overall smoking history of 7.63% of patients (95% confidence interval (CI) 3.83–12.43%) who were infected with SARS-CoV-2. Another meta-analysis with 5,960 hospitalized COVID-19 patients data suggest that current smoking pooled prevalence of 6.5% (1.4–12.6%) [11][34].
Several epidemiologic case-control and cohort studies show the strong relationship between smoking and severity on COVID-19 disease and death. Zhao et al. [12][22] identified seven studies and concluded that the risk of severe COVID-19 (Odds Ratio (OR) = 1.98; 95% CI:1.29–3.05) is nearly doubled in smokers. Zheng et al. [13] [35] analyzed the data from thirteen studies comprising 3027 patients and found that smokers had greater disease progression with COVID-19, (current smoking: OR = 2.51; 95% CI: 1.39–3.32; p = 0.0006). Baskaran et al. [14] [36] conducted a meta-analysis in 2019 which included 27 studies and 460,592 participants and found that active smokers (pooled OR = 2.17; 95% CI: 1.70–2.76), and ex-smokers (pooled OR = 1.49; 95% CI: 1.26–1.75) were more prone to develop community-acquired pneumonia compared to patients who had never smoked. A recent meta-analysis published by Patanavanich and Glantz which includes 19 peer-reviewed papers with data on smoking and COVID disease progression (17 from China, 1 from Korea, and 1 from the US) concluded that smoking is associated with more double the odds of disease progression in people with COVID-19 infections (OR = 1.91; 95% CI: 1.42–2.59; p = 0.001) [15][37]. Liu et al. [38] [16] conducted a study in a population of 78 patients with COVID-19 and found a statistically significant association between smoking and COVID-19 severity (OR = 14.28; 95% CI: 1.58–25.00; p = 0.018). Yu et al. [17] [39] who reported on a study of 70 patients, a statistically significant (OR = 16.1; 95% CI: 1.3–204.2) in a multivariate analysis examining the association between smoking and the exacerbation of pneumonia after treatment.
In contrast, several other studies could not find statistically significant link between smoking and COVID-19 severity. Chen et al. [18] [40] conducted a single center retrospective observational study in Taizhou, Zhejiang, China which included 145 patients with COVID-19. This study concluded that the more severely ill patients had a lower history of smoking when compared to less severely ill patients (7.0% vs. 11.8%, p = 0.57). In a recent study, Dong et al. [19][41] analyzed data from 11 patients with COVID-19 and concluded that there was a non-significant relationship between smoking and severity of COVID-19. More findings by Kim et al. [20][42] in a recent Korean cohort study with 28 hospitalized patients with confirmed COVID-19 in which only 5 of the patients out of 27 (18.5%) had any smoking history. Another study by Zheng et al. [21] [43] reported that only 10.9% of all patients in the study were smokers, but 6.7% were smokers in the severe/critically ill group, which is not significantly (χ2 = 0.962, p > 0.05) lower than of the ordinary group with 14.0%. They further concluded that no evidence exists that smoking protects COVID-19 patients from developing to severe disease. Furthermore, another study comprising 140 patients infected with SARS-CoV-2 in Wuhan, China, showed that only 9 (6.4%) patients had a history of smoking, and 7 of them were past smokers, which suggests that smoking history may not be a risk factor for COVID-19 disease severity [22][44].
The incomplete patient health histories may impact the significance of hospital-based studies [22][44]. In an emergency context, smoking history collection is challenging and the severity of the disease is often unclear and inconsistent throughout studies at different institutions and likely to lead to significant sampling bias [22][23][44,45]. Characteristics of hospitalized patients can also vary based on the services accessible, admission to clinics, treatment procedures, and likely other considerations not included in the studies. Further, most study analyses are based on unadjusted ORs that were either reported in the studies or calculated to account for age and other confounding factors [24][15][24,37]. Some peer-reviewed meta-analyses investigating the association between smoking and COVID-19 were also based on unadjusted ORs, but with few studies included [12][13][25][26][22,35,46,47]. All these evidences support that smoking leads to greater morbidity and mortality in COVID-19 patients.
3. Does Cessation of Tobacco Consumption Lead to Lower COVID-19 Risk?
Studies show that smokers are more prone to developing COVID-19 and more vulnerable to severe COVID-19 complications. A crucial question both to determine causality and to provide advice and effective interventions to patients is whether the risk of developing a tobacco-associated COVID-19 infection can be reduced after quitting smoking. Unfortunately, there are limited existing data on the impact of smoking cessation during the COVID-19 pandemic. Research data suggest that up to 70% of smokers have an interest in stopping smoking, but only 3–10% can do so on their own [27][28][124,125]. Smoking cessation before surgery leads to a rapid reduction in nicotine and carboxyhemoglobin a level in the bloodstream, which also provides smokers an opportunity to engage in long-term smoking cessation [29][30][126,127]. Smoking withdrawal for 4 weeks or longer has evidence of a decreased risk for COVID-19 and a lower risk of more severe complications. A recent study by Turan et al. [31][128] examining 635,265 non-cardiac surgical patients noted that current smokers had a higher likelihood of 30-day mortality (RR = 1.38; 95% CI: 1.11–1.72) and serious post-operative complications such as surgical site infection (OR = 1.30; 95% CI: 1.80–2.43), unplanned intubation (OR = 1.87; 95% CI: 1.58–2.21), pneumonia (OR = 2.09; 95% CI: 1.80–2.43), and septic shock(OR = 1.55; 95% CI: 1.29–1.87). In other meta-analysis by Wong et al. [32] [129] that included 25 studies on short term preoperative smoking cessation and post-operative complications, it was found that a minimum of 4-weeks of smoking cessation before surgery lowered the risk of respiratory complications and that cessation of at least three to four weeks also reduced wound-healing complications when compared to current smokers.
Findings also suggest that the serum half-lives of carbon monoxide and nicotine are approximately 4 h and 1 h, respectively [30][127]. Another study reported that an average carboxyhemoglobin serum level in smokers is 3.81 ± 2.17 g/dL−1 as compared to 2.95 ± 1.33 g/dL−1 in non-smokers [33][130]. It is plausible that an increase in cessation rates could help minimize community transmission of SARS-CoV-2. Evidence shows that various strategies incorporating both pharmacological and behavioral interventions during viral epidemics are better positioned to reduce smoking-related complications by encouraging cessation [34][131]. Healthcare providers should be involved in offering evidence-based pharmacological and behavioral smoking cessation interventions by remote support if in-person visits are not possible. A recent study shows that varenicline is the most effective smoking cessation pharmacotherapy followed by bupropion and nicotine patches [35][132]. In a 12-week double-blind, randomized, placebo-controlled clinical trial study of 8144 participants, varenicline improved higher abstinence rates compared with placebo (OR = 3.61; 95% CI: 3.07–4.24), nicotine patch (OR = 1.68; 95% CI: 1.46–1.93), and bupropion (OR = 1.75; 95% CI 1.52–2.01) [35][132]. Furthermore, bupropion and nicotine patch showed a trend toward higher smoking cessations rates as compared to placebo (OR = 2.07; CI: 1.75–2.45 and OR = 2.15; 95% CI: 1.82–2.54, respectively) [35][132]. Telemedicine can be employed by doctors to advise smokers about the benefits of smoking cessation. Long-term lockdowns will contribute to and increase the frequency of social distancing, and may exacerbate or even cause mental disorders, which can increase the urge to smoke; within these socially deprived populations, smoking becomes more common, which increases the risk of this population contracting COVID-19. Cessation of smoking is expected to reduce the risk of COVID-19 emerging and severe COVID-19 complications.
4. Therapeutic agent for treatment of COVID-19
At present, considering the terrible COVID-19 pandemic, there is an urgent requirement to explore the effective treatment approaches against COVID-19. Using various computational tools, genetic analyses, and protein modeling, several therapeutic strategies have been proposed using pre-existing drugs repurposed for SARS-CoV-2 therapy in an attempt to bypass the rigor of clinical trials required for novel therapeutic agents [36][37][38][39][40][156,157,158,159,160]. As a result, various potential targets, including the viral S protein, ACE-2, TMPRSS2, 3C-like protease (3CLpro), RNA-dependent RNA polymerase (RdRp), and Papain-like protease (PLpro) have been identified for repurposing pre-existing antiviral drugs and new small molecules that are under development against SARS and other coronavirus infections. Here, we will focus on the research progress of chloroquine, hydroxychloroquine, remdesivir, and lopinavir/ritonavir (Figure 14). These drugs can be used alone or in combination to combat against the virus.
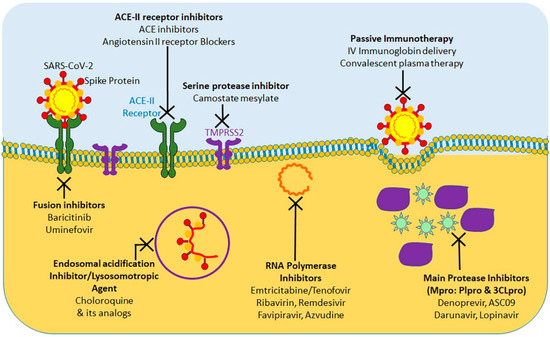
Figure 14.
The protease inhibitors, lopinavir and ritonavir (anti-retroviral drugs) have shown effectiveness against human immunodeficiency virus 1 (HIV-1) infection, SARS-CoV, MERS-CoV, and SARS-CoV-2 viruses in in vitro susceptibility models [41][42][43][49,161,162]. Protease inhibitors effectively inhibit the 3CLpro enzyme, thus posing a potentially potent therapeutic agent for controlling SARS-CoV-2 infection. To evaluate the efficacy of lopinavir/ritonavir for SARS-CoV-2 infection, a controlled clinical trial was conducted on 134 confirmed patients with novel coronavirus pneumonia. As indicated in a recent study, lopinavir and ritonavir neither improve symptoms nor affected the conversion time in respiratory tract samples [44][163]. Cao et al. [45][164] also reported that there was no observed benefit with lopinavir–ritonavir treatment compared to standard care in patients with severe COVID-19. A recent study show that lopinavir–ritonavir co-therapy along with another anti-influenza drug, oseltamivir, was reported to result in complete recovery after showing signs of COVID-19 related pneumonia [46][47][165,166]. SARS-CoV-2 and SARS-CoV RdRp share 96% sequence identity; this implies that inhibitors effective against SARS-CoV could have similar inhibitory effects against SARS-CoV-2 [48][167]. In addition, nucleoside analogs of adenine or guanine derivatives can be used against RdRp to impede viral RNA synthesis. Remdesivir (GS-5734), is a phosphoramidate prodrug of an adenine derivative, and which was used in treatments against Ebola, SARS-CoV, and MERS-CoV, as it has the potential to outcompete the proofreading ability of coronavirus exonuclease, and carries a high genetic resistance barrier [14][21][23][49][36,43,45,101]. Recent in vitro studies have shown that remdesivir effectively inhibits SARS-CoV-2 (EC50 in Vero E6 cells = 0.77 μM [50][36][11,156]. Earlier studies implied that remdesivir exhibited broad-spectrum activity against SARS-CoV-2 and similar viruses (including SARS-CoV and MERS-CoV) [36][51][156,168].
Favipiravir (T-705), is a nucleoside analogue that inhibits RdRp of RNA viruses, such as influenza virus, Ebola virus, flavivirus, chikungunya virus, norovirus, and enterovirus [52][169]. Another recent study has shown that Favipiravir (EC50 in Vero E6 cells = 61.88 μM) against SARS-CoV-2 and blocked viral infection [36][156]. A clinical study of favipiravir has shown a promising clinical efficacy in treating SARS-CoV-2 and its possibility to be safely included in future treatment plans, as it has shown both efficacy and bio-availability of the drug [53][170].
In addition, ribavirin and azvudine have shown some promising in vitro results for their efficacy against SARS-CoV-2, and it’s in vivo efficacy is currently being tested in three ongoing clinical trials [54][55][171,172].
Chloroquine and hydroxychloroquine are currently licensed for malaria, and autoimmune diseases such as lupus, blood disorder porphyria cutanea, rheumatoid arthritis and many countries have extensively used for COVID-19 treatment [56][57][173,174]. Researchers have found that Chloroquine and hydroxychloroquine have in vitro inhibitory effect against several viruses in including SARS-CoV and SARS-CoV-2 [56][58][173,175]. Based on these results, chloroquine and hydroxychloroquine are currently promoted for treatment of hospitalized COVID-19 patients in several countries [59][176]. A chloroquine-based drug was documented to inhibit SARS coronavirus fusion with the cells, by acidifying lysosomes and thereby inhibiting pH-low cathersin to optimally cleavage the S protein SARS-CoV-2 [58][175]. In addition, chloroquine has been shown to disrupt terminal ACE-2 glycosylation in the Golgi system, thus inhibiting viral penetration into host cells [60][177]. However, neither chloroquine nor hydroxychloroquine have been used in clinical setting to prevent COVID-19, because their administration is associated with significant adverse effects, such as loss of vision, nausea, stomach problems, and potential cardiovascular issues. Chloroquine and hydroxychloroquine act by accumulating in lymphocytes and macrophages and by reducing the secretion of proinflammatory cytokines, and/or by activating anti-SARS-CoV-2 CD8+T-cells [60][177]. These antimalarial agents have shown in vitro efficacy, either alone or in combination with azithromycin, against SARS-CoV-2 according to the results of several recent studies [61][62][178,179]. However, these results have been challenged by new trials with no substantial advantages from hydroxychloroquine administration even though antimalaria drugs are being tested in more than 30 randomized controlled trials [55][172].
Virus-induced immune responses leading to cytokine storm syndrome and hyperinflammation are associated with ARDS which can result in multiple organ dysfunction or death [63][149] Immunosuppressants along with antivirals play a crucial role in counteracting severe SARS-CoV-2 infection [63][149]. Several other immunomodulators and anti-inflammatory drugs are also being applied in clinical trials [55][172].
5.
Conclusion
In conclusion, a better identification of smoking and vaping histories of vulnerable individuals and their early detection, as well as targeting viral S protein, ACE-2, TMPRSS2, 3CLpro, RdRp, and PLpro and cytokine storm’ may be a significant game changer in managing the spread of this pandemic and further infections.In addition, to flatten the COVID-19 curve, staying indoors, avoiding unnecessary social contact, and bolstering the immune defense system by maintaining a healthy diet/living are highly desirable.
