Heart failure with preserved ejection fraction (HFpEF) is a condition with increasing incidence, leading to a health care problem of epidemic proportions for which no curative treatments exist. Consequently, an urge exists to better understand the pathophysiology of HFpEF. Accumulating evidence suggests a key pathophysiological role for coronary microvascular dysfunction (MVD), with an underlying mechanism of low-grade pro-inflammatory state caused by systemic comorbidities.
- heart failure with preserved ejection fraction
- microcirculation
- microvascular dysfunction
1. Introduction
2. Defining Microvascular Function and Dysfunction
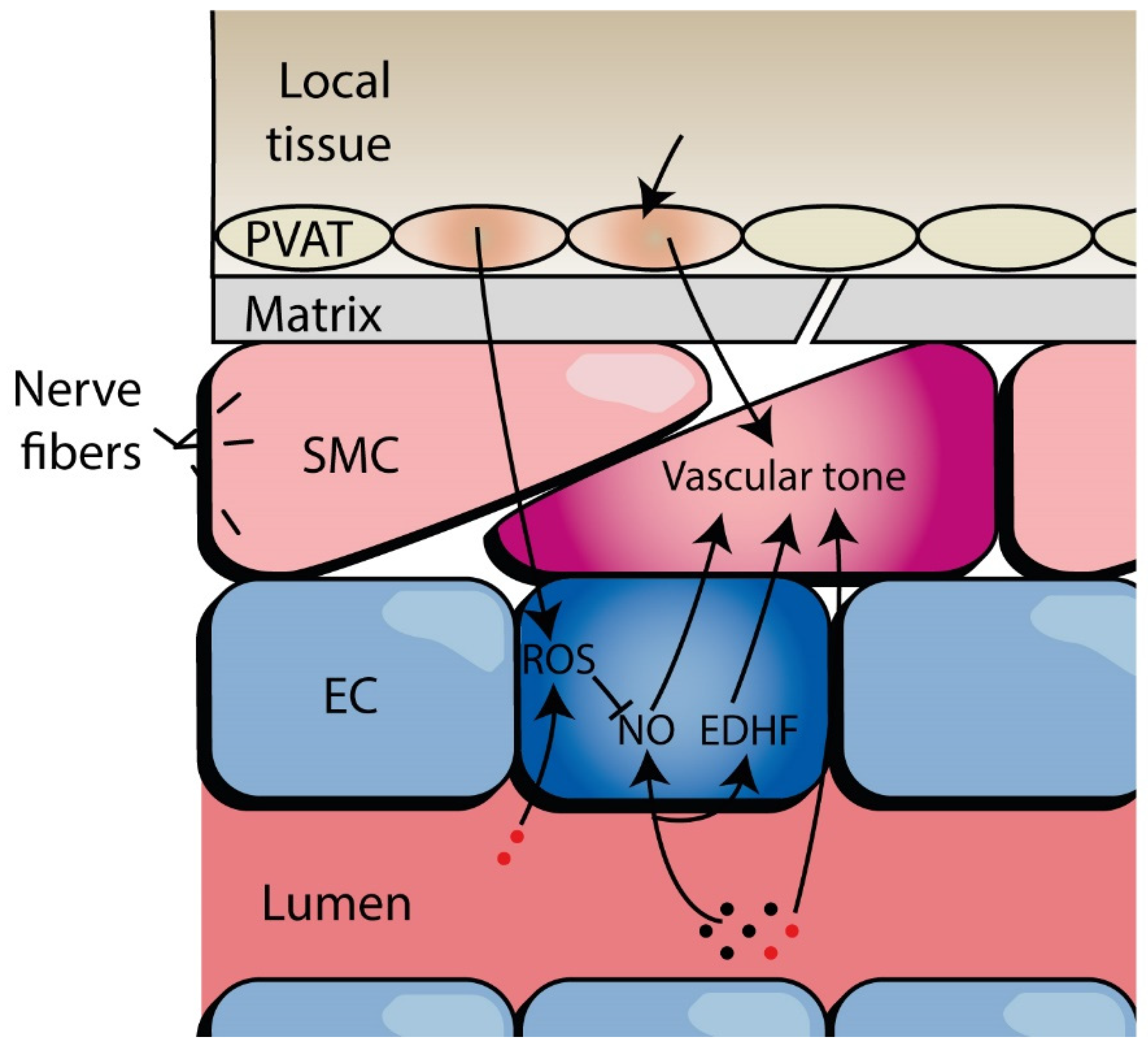
3. Evidence of Microvascular Dysfunction in HFpEF
| Study Design | HFpEF Population | Control Population | Method (Measurement) | Stimulus | Microvascular Function Assessed | Outcome (SD/IQR) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microvascular Function Assessed | Outcome (SD/IQR) | Outcome Adjusted for Confounders | |||||||||||||||
| Skin-finger | |||||||||||||||||
| Heart-autopsy | |||||||||||||||||
| Prospective | [22] | ||||||||||||||||
| Retrospective | [12 | n = 321 | Controls without HF, matched for age, sex, HT, and DM (n = 173) | Peripheral arterial tonometry (endoPAT): (RHI) | Ischemia | Hyperaemia | ] | Deceased: HFpEF (n = 124); Controls (no HF) (n = 104) |
Histology: microvessels/mm | 2 | (microvascular density)Log RHI: 0.53 ± 0.20 vs. 0.64 ± 0.20, p < 0.001 | ||||||
| Rarefaction | Microvascular density: 961 (800–1370) vs. 1316 (1148–1467), | p | < 0.0001 | Not performed, unmatched population | Prospective | [10] | n = 202 | No controls | 79 | endoPAT (RHI) | ] | Ischemia | [ | Hyperaemia | Log RHI: no absolute values reported. Correlation with CFR of R 0.21, p = 0.004 | ||
| Invasive coronary function assessment | 80] | Serum cholesterol panel | Skin, eye, heart | Function decreases with higher serum low-density lipoprotein cholesterol levels | Retrospective | [23] | n | ||||||||||
| Retrospective | [14] | = 159 | No controls | endoPAT (RHI) | Ischemia | ||||||||||||
| Hyperglycaemia | [81][82 | CAG after positive stress test: HFpEF > 65 ( | Hyperaemia | ] | n = 32); HFpEF < 65 (n = 24); Controls (n = 31) | Log RHI: 0.50 ± 0.09. Event free 0.52 ± 0.09 vs. Events 0.46 ± 0.08, | Invasive CFR and IMR | Adenosinep | Hyperaemia < 0.001 | ||||||||
| CFR: 1.94 ± 0.28 vs. 1.83 ± 0.32 vs. 3.24 ± 1.11, | Glucose tolerance test, fasting glucose, HbA1c | Skin, eye, heart | Function decreases with higher plasma glucose levels | p | ≤ 0.04 IMR: 39.2 ± 6.8 vs. 27.2 ± 6.4 vs. 18.3 ± 4.4, p ≤ 0.03 |
Age, sex, HT, DM, CKD, AF, BMI, LVMI. Unmatched controls | Prospective (cross-sectional) | [24] | |||||||||
| Hypertension | [34][36][69][83][84 | n = 62 | Controls matched for age, sex, HT, DM, dyslipidaemia and CAD (n = 64) | endoPAT (RHI) | Ischemia | Hyperaemia | RHI: 2.01 [1.64–2.42] vs. 1.70 [1.55–1.88], | ||||||||||
| Retrospective | [9] | ] | p | < 0.001 | |||||||||||||
| HFpEF ( | n = 162) | Invasive CFR and coronary blood flow (CBF) | Adenosine, acetylcholine | Hyperaemia | No absolute values reported. Mortality is increased in coronary MVD (HR 2.8–3.5). | Age, sex, BMI, DM, HT, hyperlipidaemia, smoking, Hb, creatinine, uric acid | 24-h systolic blood pressure shows the highest correlation | Skin, eye, skeletal muscle, heart | Function decreases with higher systolic blood pressure and by duration of hypertension | Prospective | [25] | ||||||
| Retrospective | [30] | n = 42 | HFpEF (n = 22); no HFpEF (nHFrEF (n = 46) |
endoPAT (RHI) | = 29) | Invasive CFR and CBFIschemia | Hyperaemia | Adenosine, acetylcholine | Hyperaemia | CFR: 2.5 ± 0.6 vs. 3.2 ± 0.7, p = 0.0003 Median CBF % increase: 1 (−35;34) vs. 64 (−4;133), pRHI: 1.77 [1.67–2.16] vs. 1.53 [1.42–1.94], | p | = 0.014. | |||||
| = 0.002 | |||||||||||||||||
| Dietary intake | [85] | Caffeine | Age, sex | Skin | Function is temporarily increased | Prospective | [26] | n = 26 | Healthy controls, matched for age and sex ( | ||||||||
| Prospective | [31] | n = 26) | endoPAT (RHI) | HFpEF with obstructive epicardial CAD (n = 38); HFpEF without epicardial CAD (nIschemia | = 37) | ||||||||||||
| Dietary intake | [86 | CAG (CFR, coronary reactivity, IMR) and MRI | Hyperaemia | ][ | Adenosine, acetylcholine | RHI interpretation from boxplots: 1.9 [1.6–2.9] vs. 1.8 [2.0–3.3], p = 0.036. No effect of exercise | |||||||||||
| Hyperaemia | 87] | CFR: 2.0(1.2–2.4) vs. 2.4(1.5–3.1), | p | = 0.06. IMR: 18(12–26) vs. 27(19–43), | p = 0.02. 24% microvascular spasm due to Acth. | Clinical characteristics are compared between groups based on coronary results. | High-fat diet | Skin, heart | Function is temporarily decreased | Prospective | [27] | ||||||
| Prospective (cross-sectional) | [13] | ||||||||||||||||
| Physical inactivity | n = 21 | [29][88][89][90] | Clinical indication for CAG: HFpEF (n = 30); Controls (n = 14)HT controls without HF (n = 19) |
24-h accelerometer, physical activity questionnaire | Healthy controls (n = 10) | endoPAT (RHI) | Ischemia | Hyperaemia | Skin, eye, skeletal muscle | Log RHI: 0.85 ± 0.42 vs. 0.92 ± 0.38 vs. 1.33 ± 0.34, p = n.s. between HFpEF and HT controls | |||||||
| Invasive CFR and IMR | Adenosine | Hyperaemia | CFR: 2.55 ± 1.60 vs. 3.84 ± 1.89, | p | = 0.024 IMR: 26.7 ± 10.3 vs. 19.7 ± 9.7, p = 0.037 |
Exploratory analysis on age, BMI, GFR, BNP, echocardiographic data, hemodynamic data. Unmatched controls | Function decreases with more physical inactivity. | Skin-arm | |||||||||
| Retrospective | [32] | ||||||||||||||||
| Obesity | [8][69][91][92] | Patients with angina presented to the ER: HFpEF (n = 155); Controls (n = 135) | Total myocardial blush grade score (TMBGS) | None, nitroglycerin | Blood flow | TMBGS: 5.6 ± 1.22 vs. 6.1 ± 1.26, p = 0.02 | Not performed, unmatched population | Waist circumference is more correlated than BMI or BSA. | Skin, eye, skeletal muscle, heart | Function decreases with increasing level of obesity | Prospective | [28] | n = 45 | HT controls, matched for age, sex and diabetic status (n = 45) | Laser Doppler flowmetry (LDF), power spectral density (PSD) of the LDF signal | None, ischemia | Vasomotion, hyperaemia |
| Sex | [93][94] | LDF PSD: lower in HFpEF, no absolute numbers reported, | p | < 0.05. | Peak blood flow (PU): 135 [104–206] vs. 177 [139–216], p = 0.03 | ||||||||||||
| Non-invasive coronary assessment | Skin, eye, skeletal muscle, heart | Effect on function depends on other confounders. | Prospective | [11] | HFpEF with CAD n = 12 | ||||||||||||
| Prospective | [33] | HFpEF (n = 19); Matched healthy controls (n = 19)HFrEF with CAD (n = 12) CAD without HF (n = 12) |
PET (C-acetate-11): myocardial blood flow (MBF) and myocardial oxygen consumption (MVOLaser Doppler imaging (LDI) coupled with transcutaneous iontophoresis of vasodilators | 2 | acetylcholine, sodium nitroprusside | Hyperaemia | )Vasodilation due to Acth: No absolute values reported. p | Dobutamine | Blood flow, hyperaemia, diffusion | MBF increase: 78% vs. 151%, p = 0.0480 MVO2 increase: 59% vs. 86%, p = 0.0079 = 0.00099 (HF vs. controls). Vasodilation due to nitroprusside: p = 0.006 (HF vs. controls) |
|||||||
| Absolute values during stress test not significantly different. | LVH, Hb. Healthy controls were matched for age and sex. | Muscle-leg | |||||||||||||||
| Retrospective | [34] | Indication for cardiac PET: HFpEF (n = 78); HT without HF (n = 112); No HF no HT (n = 186) | PET (Rb-82): global myocardial flow reserve (MFR) | Dipyridamole | Hyperaemia | MFR: 2.16 ± 0.69 vs. 2.54 ± 0.80 vs. 2.89 ± 0.70, p ≤ 0.001 | Age, sex, BMI, smoking, DM, HT, hyperlipidaemia, HT, AF, statin use. Controls matched for HT. | Prospective | [16] | n = 22 | Healthy controls, age-matched (n = 43). | Histology (skeletal muscle biopsy of thigh) | Capillary density | Capillary-to-fibre ratio: 1.35 ± 0.32 vs. 2.53 ± 1.37, p = 0.006 | |||
| Prospective | [29] | n = 7 | No controls. | Near-infrared spectroscopy: index for skeletal muscle haemoglobin oxygenation of thigh | Diffusion | Muscle deoxygenation overshoot was decreased after priming exercise, p = 0.041 | |||||||||||
| Study Design | Study Population | Method (Measurement) | Stimulus | ||||
|---|---|---|---|---|---|---|---|
| Retrospective | |||||||
| [ | |||||||
| 35 | |||||||
| ] | |||||||
| Suspected CAD: Cohort without HF ( | |||||||
| n | |||||||
| = 201) | |||||||
| PET (Rb-82): (CFR) | |||||||
| Regadenoson or dipyridamole | Hyperaemia | 18% of the patients had a HFpEF event during follow-up. Independent HR with CFR <2.0 of 2.47 (1.09–5.62) | In entire cohort: AF, CKD, troponin, LVEF, CFR, E/e’ septal | ||||
| Prospective | [36] | HFpEF (n = 25); LVH (n = 13); Controls (n = 18) |
MRI (CFR) | Adenosine | Hyperaemia | CFR: 2.21 ± 0.55 vs. 3.05 ± 0.74 vs. 3.83 ± 0.73, p ≤ 0.002 | BNP, LVEF, E/e’, LA dimension |
| Retrospective | [37] | HFpEF without events (n = 137), with events (n = 26) | MRI (CFR) | Adenosine | Hyperaemia | CFR: 2.67 ± 0.64 vs. 1.93 ± 0.38 | Not performed |
| Prospective | [38] | HFpEF (n = 6); Post MI (n = 6); Healthy controls (n = 20) | MRI: intravascular volume of basal septum (IVV) | Gadofosveset | Permeability | IVV: 0.155 ± 0.033 vs. 0.146 ± 0.038 vs. 0.135 ± 0.018, p = 0.413 | Not performed, unmatched controls |
| Prospective | [10] | HFpEF (n = 202) | Echocardiography (CFR) | Adenosine | Hyperaemia | CFR: 2.13 ± 0.51 | Age, sex, BMI, AF, DM, CAD, smoking, LV mass, 6MWT, KCCQ, urinary albumin-creatinine ratio. No controls. |
| Prospective | [39] | HFpEF (n = 77); Healthy controls (n = 30) |
Echocardiography (CFR) | Adenosine | Hyperaemia | CFR: 1.7 ± 0.2 (with MVD) vs. 3.1 ± 0.4 (no MVD) vs. 3.4 ± 0.3 (control) | Age, LAVI, LVMI, LVEF, E/e’, 6MWT distance |
3.1. Vasoreactivity in HFpEF
3.1.1. Endothelium-Dependent Vasodilation
3.1.2. Endothelium-Independent Vasodilation
| Clinical Factor | Measurement Method | Microvascular Bed Assessed | Effect on Microvascular Function | |
|---|---|---|---|---|
| Age | [34][69][72][73][74] | Skin, eye, skeletal muscle, heart | Function decreases by increasing age | |
| Hormonal status | [75][76][77][78] | Oestrogen levels, together with oestrogen receptor activity, are most accurate. Menopausal status and oral contraceptive therapy use are alternative surrogate markers. | Skin, skeletal muscle, heart | Function decreases with lower oestrogen activity |
| Hypercholesterolemia | [70][ | |||
| Smoking | ||||
| [ | ||||
| 74 | ||||
| ] | ||||
| [ | ||||
| 95 | ||||
| ] | Self-reported use | Skin, eye, heart | Function decreases with smoking and more pack years. |
3.2. Other alterations of the microcirculation in HFpEF
Elaboration on these topics are reviewed elsewhere [96]. Briefly, capillary rarefaction of vascular beds in the upper legs and the heart, as well as biomarkers of microvascular function such as vascular cell adhesion molecule 1 (VCAM-1), have been reported in HFpEF compared to controls. Moreover, tissues such as adipocytes and cardiomyocytes have alterations in HFpEF compared to controls.4. Systemic Microvascular Dysfunction in HFpEF
Systemic MVD is present in HFpEF, based on interpretation of abundant data from many correlational studies that show impairments in microvascular function, both endothelium-dependent and endothelium-independent, in different vascular beds. MVD should be seen as a continuum between function and dysfunction, which can influence HFpEF and comorbidity progression, and vice versa. Hitherto, due to a lack of clear causative evidence, it remains unknown how systemic MVD could drive HFpEF.
Furthermore, HFpEF patients unequally show different elements of MVD, which might reflect different underlying mechanisms and therapeutic targets. Future research on MVD and HFpEF is, therefore, needed to uncover the true diagnostic and therapeutic value of microvascular assessments. This will require more uniformity and confounder considerations in study design, analyses, and reporting. However, the incorporation of peripheral microvascular assessments is feasible and should be considered in clinical HFpEF trials.
References
- Reddy, Y.N.; Borlaug, B.A. Heart Failure With Preserved Ejection Fraction. Curr. Probl. Cardiol. 2016, 41, 145–188.
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975.
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709.
- Senni, M.; Caravita, S.; Paulus, W.J. Do Existing Definitions Identify Subgroup Phenotypes or Reflect the Natural History of Heart Failure With Preserved Ejection Fraction? Circulation 2019, 140, 366–369.
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory markers and incident heart failure risk in older adults: The Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137.
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271.
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschope, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324.
- Yang, J.H.; Obokata, M.; Reddy, Y.N.V.; Redfield, M.M.; Lerman, A.; Borlaug, B.A. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 432–441.
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxen, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450.
- Balmain, S.; Padmanabhan, N.; Ferrell, W.R.; Morton, J.J.; McMurray, J.J. Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur. J. Heart Fail. 2007, 9, 865–871.
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559.
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A.P.; Nathan, S.; Butler, J.; Davidson, C.J.; Fearon, W.F.; et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1033–H1042.
- Xu, Z.; Gu, H.P.; Gu, Y.; Sun, W.; Yu, K.; Zhang, X.W.; Kong, X.Q. Increased index of microcirculatory resistance in older patients with heart failure with preserved ejection fraction. J. Geriatr. Cardiol. 2018, 15, 687–694.
- Sandesara, P.B.; O’Neal, W.T.; Kelli, H.M.; Samman-Tahhan, A.; Hammadah, M.; Quyyumi, A.A.; Sperling, L.S. The Prognostic Significance of Diabetes and Microvascular Complications in Patients With Heart Failure With Preserved Ejection Fraction. Diabetes Care 2018, 41, 150.
- Kitzman, D.W.; Nicklas, B.; Kraus, W.E.; Lyles, M.F.; Eggebeen, J.; Morgan, T.M.; Haykowsky, M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1364–H1370.
- Houben, A.J.H.M.; Stehouwer, C.D.A. Microvascular dysfunction: Determinants and treatment, with a focus on hyperglycemia. Endocr. Metab. Sci. 2021, 2, 100073.
- Houben, A.; Martens, R.J.H.; Stehouwer, C.D.A. Assessing Microvascular Function in Humans from a Chronic Disease Perspective. J. Am. Soc. Nephrol. 2017, 28, 3461–3472.
- Segal, S.S. Integration and Modulation of Intercellular Signaling Underlying Blood Flow Control. J. Vasc. Res. 2015, 52, 136–157.
- Secomb, T.W.; Pries, A.R. The microcirculation: Physiology at the mesoscale. J. Physiol. 2011, 589, 1047–1052.
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium. Circ. Res. 2007, 100, 158–173.
- Akiyama, E.; Sugiyama, S.; Matsuzawa, Y.; Konishi, M.; Suzuki, H.; Nozaki, T.; Ohba, K.; Matsubara, J.; Maeda, H.; Horibata, Y.; et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 1778–1786.
- Matsue, Y.; Suzuki, M.; Nagahori, W.; Ohno, M.; Matsumura, A.; Hashimoto, Y.; Yoshida, K.; Yoshida, M. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. 2013, 168, 36–40.
- Yamamoto, E.; Hirata, Y.; Tokitsu, T.; Kusaka, H.; Sakamoto, K.; Yamamuro, M.; Kaikita, K.; Watanabe, H.; Hokimoto, S.; Sugiyama, S.; et al. The pivotal role of eNOS uncoupling in vascular endothelial dysfunction in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. 2015, 190, 335–337.
- Waku, R.; Tokoi, S.; Toyoda, S.; Kitahara, K.; Naganuma, J.; Yazawa, H.; Sakuma, M.; Abe, S.; Nakajima, T.; Inoue, T. Flow-Mediated Vasodilation and Reactive Hyperemia Index in Heart Failure with Reduced or Preserved Ejection Fraction. Tohoku J. Exp. Med. 2020, 252, 85–93.
- Gevaert, A.B.; Beckers, P.J.; Van Craenenbroeck, A.H.; Lemmens, K.; Van De Heyning, C.M.; Heidbuchel, H.; Vrints, C.J.; Van Craenenbroeck, E.M. Endothelial dysfunction and cellular repair in heart failure with preserved ejection fraction: Response to a single maximal exercise bout. Eur. J. Heart Fail. 2019, 21, 125–127.
- Borlaug, B.A.; Olson, T.P.; Lam, C.S.; Flood, K.S.; Lerman, A.; Johnson, B.D.; Redfield, M.M. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2010, 56, 845–854.
- Marechaux, S.; Samson, R.; van Belle, E.; Breyne, J.; de Monte, J.; Dedrie, C.; Chebai, N.; Menet, A.; Banfi, C.; Bouabdallaoui, N.; et al. Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. J. Card. Fail. 2016, 22, 3–11.
- Boyes, N.G.; Eckstein, J.; Pylypchuk, S.; Marciniuk, D.D.; Butcher, S.J.; Lahti, D.S.; Dewa, D.M.K.; Haykowsky, M.J.; Wells, C.R.; Tomczak, C.R. Effects of heavy-intensity priming exercise on pulmonary oxygen uptake kinetics and muscle oxygenation in heart failure with preserved ejection fraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R199–R209.
- Ahmad, A.; Corban, M.T.; Toya, T.; Verbrugge, F.H.; Sara, J.D.; Lerman, L.O.; Borlaug, B.A.; Lerman, A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 765–772.
- Rush, C.J.; Berry, C.; Oldroyd, K.G.; Rocchiccioli, J.P.; Lindsay, M.M.; Touyz, R.M.; Murphy, C.L.; Ford, T.J.; Sidik, N.; McEntegart, M.B.; et al. Prevalence of Coronary Artery Disease.e and Coronary Microvascular Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2021, 6, 1130–1143.
- Sucato, V.; Evola, S.; Novo, G.; Sansone, A.; Quagliana, A.; Andolina, G.; Assennato, P.; Novo, S. Angiographic Evaluation of Coronary Microvascular Dysfunction in Patients with Heart Failure and Preserved Ejection Fraction. Microcirculation 2015, 22, 528–533.
- AbouEzzeddine, O.F.; Kemp, B.J.; Borlaug, B.A.; Mullan, B.P.; Behfar, A.; Pislaru, S.V.; Fudim, M.; Redfield, M.M.; Chareonthaitawee, P. Myocardial Energetics in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e006240.
- Srivaratharajah, K.; Coutinho, T.; deKemp, R.; Liu, P.; Haddad, H.; Stadnick, E.; Davies, R.A.; Chih, S.; Dwivedi, G.; Guo, A.; et al. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002562.
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 840–849.
- Kato, S.; Saito, N.; Kirigaya, H.; Gyotoku, D.; Iinuma, N.; Kusakawa, Y.; Iguchi, K.; Nakachi, T.; Fukui, K.; Futaki, M.; et al. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2016, 5.
- Kato, S.; Fukui, K.; Kodama, S.; Azuma, M.; Nakayama, N.; Iwasawa, T.; Kimura, K.; Tamura, K.; Utsunomiya, D. Cardiovascular magnetic resonance assessment of coronary flow reserve improves risk stratification in heart failure with preserved ejection fraction. J. Cardiovasc. Magn. Reson. 2021, 23, 112.
- Masci, P.G.; Pavon, A.G.; Berchier, G.; Schwitter, J. Probing the intravascular and interstitial compartments of remodeled myocardium in heart failure patients with preserved and reduced ejection fraction: A CMR study. BMC Med. Imaging 2019, 19, 1.
- Mahfouz, R.A.; Gouda, M.; Abdelhamid, M. Relation of microvascular dysfunction and exercise tolerance in patients with heart failure with preserved ejection fraction. Echocardiography 2020, 37, 1192–1198.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Bivalacqua, T.J.; Champion, H.C.; Lambert, D.G.; Kadowitz, P.J. Vasodilator responses to adenosine and hyperemia are mediated by A1 and A2 receptors in the cat vascular bed. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1696–R1709.
- Adapala, R.K.; Talasila, P.K.; Bratz, I.N.; Zhang, D.X.; Suzuki, M.; Meszaros, J.G.; Thodeti, C.K. PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H757–H765.
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 Channels Mediate Cyclic Strain–Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ. Res. 2009, 104, 1123–1130.
- Wilson, R.F.; Wyche, K.; Christensen, B.V.; Zimmer, S.; Laxson, D.D. Effects of adenosine on human coronary arterial circulation. Circulation 1990, 82, 1595–1606.
- Duncker, D.J.; Bache, R.J. Regulation of Coronary Blood Flow During Exercise. Physiol. Rev. 2008, 88, 1009–1086.
- Tadamura, E.; Iida, H.; Matsumoto, K.; Mamede, M.; Kubo, S.; Toyoda, H.; Shiozaki, T.; Mukai, T.; Magata, Y.; Konishi, J. Comparison of myocardial blood flow during dobutamine-atropine infusion with that after dipyridamole administration in normal men. J. Am. Coll. Cardiol. 2001, 37, 130–136.
- Prior, J.O.; Quiñones, M.J.; Hernandez-Pampaloni, M.; Facta, A.D.; Schindler, T.H.; Sayre, J.W.; Hsueh, W.A.; Schelbert, H.R. Coronary Circulatory Dysfunction in Insulin Resistance, Impaired Glucose Tolerance, and Type 2 Diabetes Mellitus. Circulation 2005, 111, 2291–2298.
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaëlsson, E.; Lindstedt, E.-L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1448–1457.
- Hage, C.; Michaëlsson, E.; Kull, B.; Miliotis, T.; Svedlund, S.; Linde, C.; Donal, E.; Daubert, J.-C.; Gan, L.-M.; Lund, L.H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail. 2020, 7, 1534–1546.
- Nagele, M.P.; Barthelmes, J.; Ludovici, V.; Cantatore, S.; von Eckardstein, A.; Enseleit, F.; Luscher, T.F.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 2018, 39, 47–56.
- Mimoun, L.; Massin, P.; Steg, G. Retinal microvascularisation abnormalities and cardiovascular risk. Arch. Cardiovasc. Dis. 2009, 102, 449–456.
- Cheung, N.; Bluemke, D.A.; Klein, R.; Sharrett, A.R.; Islam, F.M.; Cotch, M.F.; Klein, B.E.; Criqui, M.H.; Wong, T.Y. Retinal arteriolar narrowing and left ventricular remodeling: The multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2007, 50, 48–55.
- Chandra, A.; Seidelmann, S.B.; Claggett, B.L.; Klein, B.E.; Klein, R.; Shah, A.M.; Solomon, S.D. The association of retinal vessel calibres with heart failure and long-term alterations in cardiac structure and function: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. J. Heart Fail. 2019, 21, 1207–1215.
- Wong, T.Y.; Rosamond, W.; Chang, P.P.; Couper, D.J.; Sharrett, A.R.; Hubbard, L.D.; Folsom, A.R.; Klein, R. Retinopathy and risk of congestive heart failure. JAMA 2005, 293, 63–69.
- Tromp, J.; Lim, S.L.; Tay, W.T.; Teng, T.-H.K.; Chandramouli, C.; Ouwerkerk, W.; Wander, G.S.; Sawhney, J.P.S.; Yap, J.; MacDonald, M.R.; et al. Microvascular Disease in Patients With Diabetes With Heart Failure and Reduced Ejection Versus Preserved Ejection Fraction. Diabetes Care 2019, 42, 1792.
- Mordi, I.R.; Tee, A.; Palmer, C.N.; McCrimmon, R.J.; Doney, A.S.F.; Lang, C.C. Microvascular disease and heart failure with reduced and preserved ejection fraction in type 2 diabetes. ESC Heart Fail. 2020, 7, 1168–1177.
- Wong, T.Y.; Islam, F.M.A.; Klein, R.; Klein, B.E.K.; Cotch, M.F.; Castro, C.; Sharrett, A.R.; Shahar, E. Retinal Vascular Caliber, Cardiovascular Risk Factors, and Inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA). Investig. Ophthalmol. Vis. Sci. 2006, 47, 2341–2350.
- Gutterman, D.D.; Chabowski, D.S.; Kadlec, A.O.; Durand, M.J.; Freed, J.K.; Ait-Aissa, K.; Beyer, A.M. The Human Microcirculation: Regulation of Flow and Beyond. Circ. Res. 2016, 118, 157–172.
- Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007, 100, 174–190.
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Naka, K. Endothelial dysfunction and heart failure: A review of the existing bibliography with emphasis on flow mediated dilation. JRSM Cardiovasc. Dis. 2019, 8, 2048004019843047.
- Rosenberry, R.; Trojacek, D.; Chung, S.; Cipher, D.J.; Nelson, M.D. Interindividual differences in the ischemic stimulus and other technical considerations when assessing reactive hyperemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R530–R538.
- Moerland, M.; Kales, A.J.; Schrier, L.; van Dongen, M.G.J.; Bradnock, D.; Burggraaf, J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int. J. Vasc. Med. 2012, 2012, 904141.
- Jakubowski, M.; Turek-Jakubowska, A.; Szahidewicz-Krupska, E.; Gawrys, K.; Gawrys, J.; Doroszko, A. Profiling the endothelial function using both peripheral artery tonometry (EndoPAT) and Laser Doppler Flowmetry (LD) - Complementary studies or waste of time? Microvasc. Res. 2020, 130, 104008.
- Fukumoto, K.; Takemoto, Y.; Norioka, N.; Takahashi, K.; Namikawa, H.; Tochino, Y.; Shintani, A.; Yoshiyama, M.; Shuto, T. Predictors of the effects of smoking cessation on the endothelial function of conduit and digital vessels. Hypertens. Res. 2021, 44, 63–70.
- Bo’, C.D.; Campolo, J.; Porrini, M.; Fracassetti, D.; Parolini, M.; Klimis-Zacas, D.; Riso, P. Acute cigarette smoking impairs microvascular function in young moderate smokers: A potential model for studying vasoactive properties of food bioactives. PharmaNutrition 2014, 2, 1–7.
- Kiowski, W.; Linder, L.; Stoschitzky, K.; Pfisterer, M.; Burckhardt, D.; Burkart, F.; Bühler, F.R. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced.d vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation 1994, 90, 27–34.
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295.
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008, 117, 2467–2474.
- Muris, D.M.; Houben, A.J.; Kroon, A.A.; Henry, R.M.; van der Kallen, C.J.; Sep, S.J.; Koster, A.; Dagnelie, P.C.; Schram, M.T.; Stehouwer, C.D. Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: The Maastricht Study. J. Hypertens. 2014, 32, 2439–2449.
- Quyyumi, A.A.; Dakak, N.; Andrews, N.P.; Gilligan, D.M.; Panza, J.A.; Cannon, R.O., 3rd. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation 1995, 92, 320–326.
- Mombouli, J.V.; Vanhoutte, P.M. Endothelial dysfunction: From physiology to therapy. J. Mol. Cell Cardiol. 1999, 31, 61–74.
- van Sloten, T.T.; Czernichow, S.; Houben, A.J.; Protogerou, A.D.; Henry, R.M.; Muris, D.M.; Schram, M.T.; Sep, S.J.; Dagnelie, P.C.; van der Kallen, C.J.; et al. Association Between Arterial Stiffness and Skin Microvascular Function: The SUVIMAX2 Study and The Maastricht Study. Am. J. Hypertens. 2015, 28, 868–876.
- Haykowsky, M.J.; Kouba, E.J.; Brubaker, P.H.; Nicklas, B.J.; Eggebeen, J.; Kitzman, D.W. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am. J. Cardiol. 2014, 113, 1211–1216.
- Myers, C.E.; Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Wong, T.Y.; Klein, B.E. Determinants of retinal venular diameter: The Beaver Dam Eye Study. Ophthalmology 2012, 119, 2563–2571.
- Arnal, J.F.; Fontaine, C.; Billon-Gales, A.; Favre, J.; Laurell, H.; Lenfant, F.; Gourdy, P. Estrogen receptors and endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1506–1512.
- Maturana, M.A.; Irigoyen, M.C.; Spritzer, P.M. Menopause, estrogens, and endothelial dysfunction: Current concepts. Clinics 2007, 62, 77–86.
- Sickinghe, A.A.; Korporaal, S.J.A.; den Ruijter, H.M.; Kessler, E.L. Estrogen Contributions to Microvascular Dysfunction Evolving to Heart Failure With Preserved Ejection Fraction. Front. Endocrinol. 2019, 10, 442.
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832.
- Nagele, M.P.; Barthelmes, J.; Ludovici, V.; Cantatore, S.; Frank, M.; Ruschitzka, F.; Flammer, A.J.; Sudano, I. Retinal microvascular dysfunction in hypercholesterolemia. J. Clin. Lipidol. 2018, 12, 1523–1531.e2.
- Zeiher, A.M.; Drexler, H.; Wollschläger, H.; Just, H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 1991, 83, 391–401.
- Sorensen, B.M.; Houben, A.; Berendschot, T.; Schouten, J.; Kroon, A.A.; van der Kallen, C.J.H.; Henry, R.M.A.; Koster, A.; Reesink, K.D.; Dagnelie, P.C.; et al. Hyperglycemia Is the Main Mediator of Prediabetes- and Type 2 Diabetes-Associated Impairment of Microvascular Function: The Maastricht Study. Diabetes Care 2017, 40, e103–e105.
- Fujimoto, K.; Hozumi, T.; Watanabe, H.; Tokai, K.; Shimada, K.; Yoshiyama, M.; Homma, S.; Yoshikawa, J. Acute hyperglycemia induced by oral glucose loading suppresses coronary microcirculation on transthoracic Doppler echocardiography in healthy young adults. Echocardiography 2006, 23, 829–834.
- Papanek, P.E.; Rieder, M.J.; Lombard, J.H.; Greene, A.S. Gender-specific protection from microvessel rarefaction in female hypertensive rats. Am. J. Hypertens. 1998, 11, 998–1005.
- Noon, J.P.; Walker, B.R.; Webb, D.J.; Shore, A.C.; Holton, D.W.; Edwards, H.V.; Watt, G.C. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J. Clin. Investig. 1997, 99, 1873–1879.
- Melik, Z.; Princi, T.; Grill, V.; Cankar, K. The effect of caffeine on cutaneous postocclusive reactive hyperaemia. PLoS ONE 2019, 14, e0214919.
- van Haare, J.; Kooi, M.E.; Vink, H.; Post, M.J.; van Teeffelen, J.J.W.; Slenter, J.; Munts, C.; Cobelens, H.; Strijkers, G.J.; Koehn, D.; et al. Early impairment of coronary microvascular perfusion capacity in rats on a high fat diet. Cardiovasc. Diabetol. 2015, 14, 150.
- Maranhao, P.A.; de Souza, M.; Panazzolo, D.G.; Nogueira Neto, J.F.; Bouskela, E.; Kraemer-Aguiar, L.G. Metabolic Changes Induced by High-Fat Meal Evoke Different Microvascular Responses in Accordance with Adiposity Status. Biomed. Res. Int. 2018, 2018, 5046508.
- Demiot, C.; Dignat-George, F.; Fortrat, J.O.; Sabatier, F.; Gharib, C.; Larina, I.; Gauquelin-Koch, G.; Hughson, R.; Custaud, M.A. WISE 2005: Chronic bed rest impairs microcirculatory endothelium in women. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3159–H3164.
- Lanting, S.M.; Johnson, N.A.; Baker, M.K.; Caterson, I.D.; Chuter, V.H. The effect of exercise training on cutaneous microvascular reactivity: A systematic review and meta-analysis. J. Sci. Med. Sport 2017, 20, 170–177.
- Streese, L.; Khan, A.W.; Deiseroth, A.; Hussain, S.; Suades, R.; Tiaden, A.; Kyburz, D.; Cosentino, F.; Hanssen, H. High-intensity interval training modulates retinal microvascular phenotype and DNA methylation of p66Shc gene: A randomized controlled trial (EXAMIN AGE). Eur. Heart J. 2020, 41, 1514–1519.
- Shankar, A.; Sabanayagam, C.; Klein, B.E.K.; Klein, R. Retinal Microvascular Changes and the Risk of Developing Obesity: Population-Based Cohort Study. Microcirculation 2011, 18, 655–662.
- Joris, P.J.; Plat, J.; Kusters, Y.H.; Houben, A.J.; Stehouwer, C.C.D.; Schalkwijk, C.G.; Mensink, R.P. Diet-induced weight loss improves not only cardiometabolic risk markers but also markers of vascular function: A randomized controlled trial in abdominally obese men. Am. J. Clin. Nutr. 2017, 105, 23–31.
- Huxley, V.H.; Kemp, S.S. Sex-Specific Characteristics of the Microcirculation. Adv. Exp. Med. Biol. 2018, 1065, 307–328.
- Kobayashi, Y.; Fearon, W.F.; Honda, Y.; Tanaka, S.; Pargaonkar, V.; Fitzgerald, P.J.; Lee, D.P.; Stefanick, M.; Yeung, A.C.; Tremmel, J.A. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1433–1441.
- Rooks, C.; Faber, T.; Votaw, J.; Veledar, E.; Goldberg, J.; Raggi, P.; Quyyumi, A.A.; Bremner, J.D.; Vaccarino, V. Effects of smoking on coronary microcirculatory function: A twin study. Atherosclerosis 2011, 215, 500–506.
- Jerremy Weerts; Sanne G. J. Mourmans; Arantxa Barandiarán Aizpurua; Blanche L. M. Schroen; Christian Knackstedt; Etto Eringa; Alfons J. H. M. Houben; Vanessa P. M. van Empel; The Role of Systemic Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. Biomolecules 2022, 12, 278, 10.3390/biom12020278.
