Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Philip Mark Mullineaux and Version 2 by Dean Liu.
Hydrogen peroxide (H2O2), generated during photosynthesis, is proposed to both initiate and transduce a retrograde signal in response to photoinhibitory light intensities.
- retrograde signalling
- chloroplasts
- nucleus
- endoplasmic reticulum
1. Introduction
Chloroplast-to-nucleus (retrograde) signalling is an important part of plants’ capacity to sense and act upon changes in their environment, especially those that require eventual adjustments to photosynthetic capacity. The ability to coordinate immediate and longer-term responses to environmental perturbations occurs at the cellular, tissue and whole plant (systemic) level [1][2][3][4][5][6][7][1,2,3,4,5,6,7]. A particularly active area within this research sphere is the quest to identify the precise signalling routes between chloroplasts and the nucleus. Several signalling pathways and signal initiators and transducers have been identified and continue to attract attention, although there are undoubtedly many more to be uncovered [8][9][10][11][12][13][8,9,10,11,12,13].
The close association of a proportion of a cell’s chloroplast complement with its nucleus is a feature of all plant species so far examined [11][14][15][11,14,15]. More recently, this relationship has received growing attention since the juxtaposition of a subset of chloroplasts with the nucleus is suggested to be a crucial feature in the communication and coordination of highly complex processes between these organelles in response to developmental and environmental cues. [4][11][16][17][18][4,11,16,17,18]. Since some signalling molecules could originate from multiple cellular sources, the close association between the nucleus and a subset of chloroplasts may provide the necessary specificity for retrograde signal transduction. Conversely, if no discrimination between the origins of such molecules was accommodated, then using them as signal transducers from the chloroplast would not provide any specificity [11][15][11,15]. The molecule where this argument is most pertinent and will be the example used in this essay, is hydrogen peroxide (H2O2) whose origin from different subcellular sources produces differential gene expression patterns, which implies that there is an associated signalling specificity [19][20][21][22][19,20,21,22].
In the context of retrograde signalling, specificity could be achieved by conversion of the oxidising equivalent from H2O2 to another molecule in the chloroplast [2][23][24][25][2,23,24,25]. While this does indeed occur, observations also suggest that H2O2 can also be the transducing signal from chloroplasts to the nucleus [4][16][4,16]. In higher plants, the movement of H2O2 between chloroplasts and the nucleus has been studied in Nicotiana benthamiana (Nb) epidermal pavement cells. This tissue is readily accessible for monitoring changes in the oxidation state of transiently expressed genetically encoded H2O2-reporting fluorescent biosensor proteins using confocal laser scanning microscopy [4][16][26][4,16,26]. Important for interpretation of responses to some environmental stresses is that Nb epidermal pavement cells are photosynthetic [4]. The H2O2 that accumulates in Nb chloroplasts in these studies arises in response to increased light intensity or to pathogen effector triggered immunity [4][16][27][4,16,27]. However, a wide range of environmental challenges cause changes in H2O2 levels in other subcellular compartments including the peroxisome, mitochondrion, cytosol and the plasma membrane [5][13][22][28][29][30][5,13,22,28,29,30]. Therefore, chloroplast-nucleus association is proposed to be relevant in determining how H2O2 secreted from chloroplasts [31] could be specific in the transduction of an oxidising signal to the nucleus [4][11][15][4,11,15].
2. H2O2, Aquaporins and the Route to the Nucleus
From the above considerations, it can be proposed that there is close association between some of a cell’s complement of chloroplasts and the nucleus, which would also involve both organelles tied into the cytoskeleton with the strength of the connections determined by tethering through MCS. More precisely, for H2O2 to travel from the chloroplast stroma to the nucleus then it must not only cross the chloroplast double envelope, but also the outer and then inner nuclear membrane separated by the perinuclear space.
The movement of H2O2 across membranes is considered to occur by diffusion down a concentration gradient facilitated by membrane intrinsic proteins (aquaporins; AQPs; reviewed by Bienert and Chaumont [32][66]). However, H2O2 diffusion into red blood cells is not facilitated by AQPs but by an unknown membrane protein or through the lipid fraction [33][67] raising the possibility of AQP-independent means of transporting H2O2 between cellular compartments. This is despite physico-chemical considerations concluding that simple diffusion of H2O2 across membranes can be disregarded [32][33][66,67]. Instead, all AQPs that transport H2O may also transport H2O2, although there are differences in the efficiency of how individual AQP isoforms discriminate between these two molecules [32][34][35][66,68,69]. Assuming a uni-directional movement of signal-transducing H2O2 to the nucleus from attached chloroplasts, then its journey would include crossing the chloroplast envelope membranes (Figure 1). Isolated chloroplasts exposed to high light intensities secrete H2O2 into their medium [31] and this is blocked by the AQP inhibitor acetazolamide [36][70]. Of the 35 AQPs in Arabidopsis [37][71], up to 5 may be present in the chloroplast. Of these, at least two isoforms of the tonoplast intrinsic protein (TIP1;1 and TIP1;2) AQP family and one of the plasma membrane intrinsic proteins, PIP2a, may span the inner chloroplast envelope membrane [38][39][40][72,73,74]. Therefore, the current evidence strongly suggests that AQPs are the exit route out of the chloroplast for H2O2.
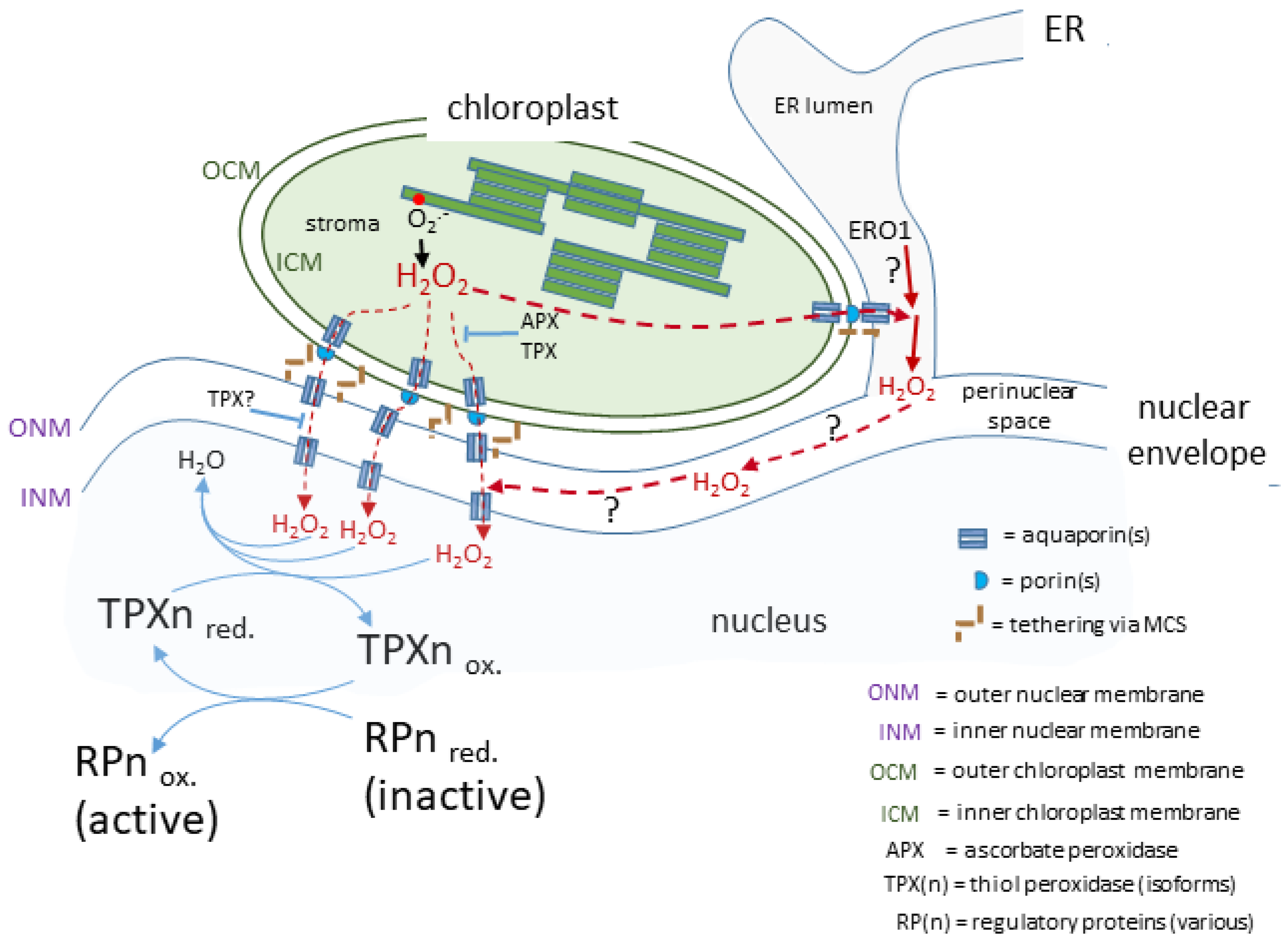

Figure 1. A proposed route for a transducing H2O2 retrograde signal. In this case, the chloroplasts and nucleus are in close association linked by the nuclear envelope and possibly influenced by H2O2 produced in the ER lumen. The H2O2 generated by photosynthetic electron transport passes through membranes facilitated by aquaporins and arrives in the nucleus to transfer its oxidising equivalents to a redox relay network ultimately leading to the activation of a range of diverse regulatory proteins, which may act in the nucleus or migrate to other subcellular sites.
The likelihood of very close contact between the chloroplast envelope and the outer nuclear envelope (see above) could include a localised increased concentration in microdomains at or near MCS and, if there is close proximity of further AQPs in the outer nuclear membrane, this would facilitate the transfer of H2O2 to the perinuclear space. Mitochondrial-ER MCS in animal cells form an environment where H2O2 does indeed concentrate in microdomains either side of the mitochondrial envelope [41][75]. It can be surmised that an analogous arrangement around chloroplast-outer nuclear/ER membrane could exist and certainly H2O2 microdomains have been observed associated with Nb epidermal chloroplasts [4]. Once in the perinuclear space, H2O2 would be in an oxidising environment (see following section) and therefore would have time to diffuse to the vicinity of any AQPs located on the inner nuclear membrane for its entry into the nucleus.
It should be emphasised that these considerations on the route from attached chloroplast to nucleus is informed speculation (Figure 12) based on the more complete information available from other eukaryotic cells. Whether this route for H2O2 actually exists in plant cells awaits experimental investigation.
3. H2O2 in the Perinuclear Space and ER Lumen and Its Impact on Retrograde Signalling
In animal cells, the ER lumen is regarded, along with mitochondria and peroxisomes, as a major source of H2O2 for signalling [34][42][43][44][68,76,77,78]. These organelles are often found in very close proximity to each other and may secrete H2O2 into a shared microdomain in which proteins involved in further transducing the oxidising signal are also present. The cooperation between these three compartments to form a cytosol-located H2O2 microdomain has been termed the “redoxosome” [44][78]. A redoxosome for these same organelles but also including chloroplasts has been suggested as possible in plant cells, but this suggestion remains unexplored [45][79]. It has been proposed that in animal cells, the directing of H2O2 to the redoxosome ensures that it does not accumulate in the nucleus and cause oxidative damage there. However, plant cells subjected to environmental stress can accumulate chloroplast-sourced H2O2 in their nucleus [4][16][4,16]. This suggests that the organisation of the spatial components of H2O2-mediated retrograde signalling may differ from those involving non-plastid organelles, which may share a degree of conservation across the Eukarya.
The midpoint redox potential of the reduced glutathione-glutathione disulphide (GSH-GSSG) couple (EGSH) in the ER lumen is −208 ±4 mV, which is more oxidising than that of the cytosol at ca. −320 mV in animal cells [46][80]. However, very recent in vivo measurements conducted on Arabidopsis ER suggest a slightly more reducing EGSH of −241 mV [47][81]. Irrespective of these differences between animal and plant cells, the ER lumen environment allows the chaperone-catalysed oxidative folding of proteins to occur that requires molecular oxygen (O2) and from which H2O2 arises (Figure 2). This is a highly conserved process in all eukaryotic cells. Oxidative stress in the ER is caused when this protein folding activity exceeds the capacity of the lumen antioxidant system to remove the H2O2 formed. GLUTATHIONE PEROXIDASE7 (GPX7), GPX8 and PEROXIREDOXIN4 (PRDX4) scavenge the H2O2 generated by the ER oxidoreductase1 (ERO1)-catalysed oxidation of the PROTEIN DISULPHIDE ISOMERASE (PDI) isoforms (Figure 2). Despite their names, GPX7 and GPX8 use reduced PDI isoforms as electron donors and not GSH [43][48][77,82]. There are also additional ERO1-independent means of generating H2O2 [49][83].
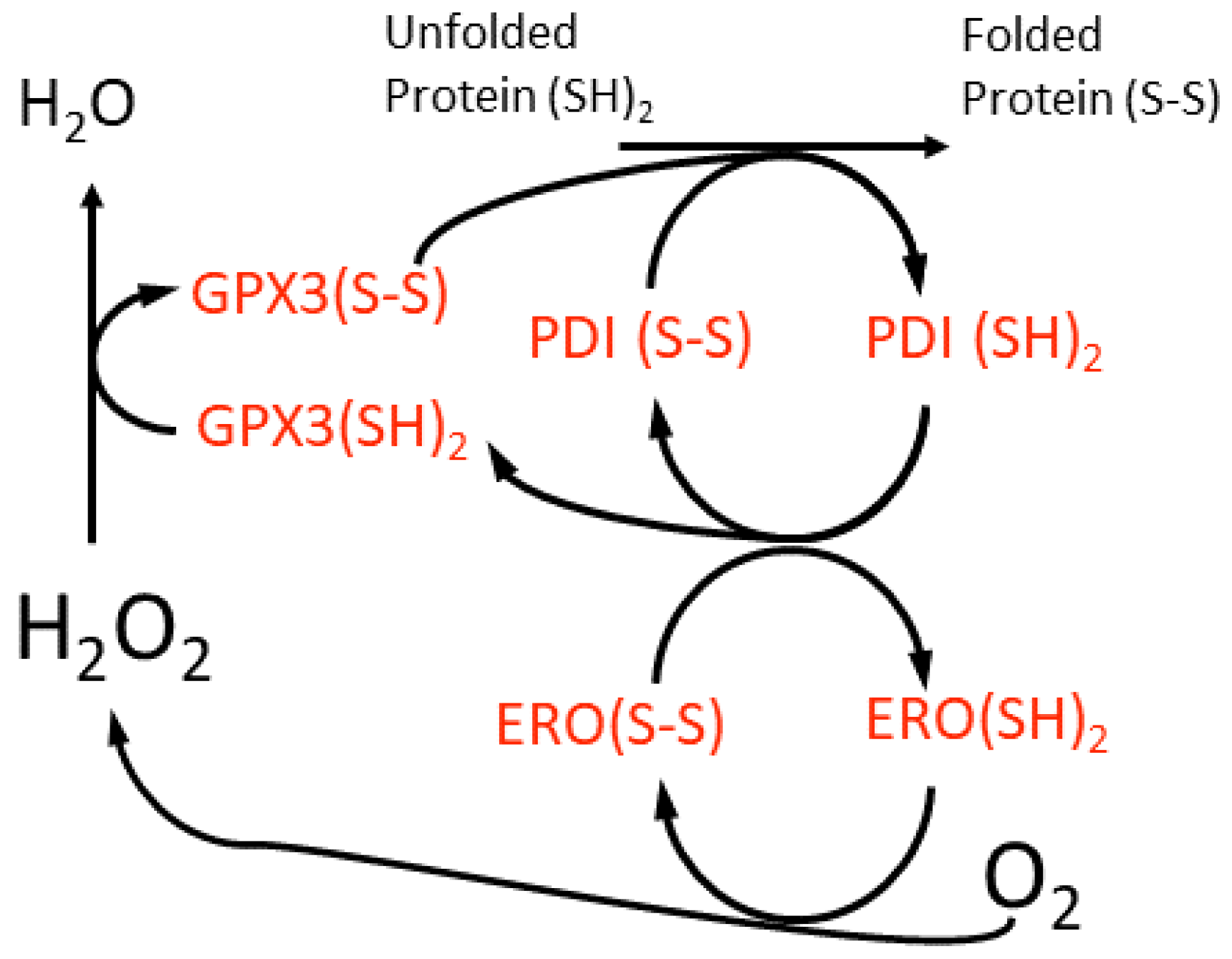

Figure 2. A scheme for the oxidative folding of proteins in the plant cell ER lumen and the generation of H2O2 by a luminal ER oxidase (ERO). This H2O2 may be scavenged by an ER glutathione peroxidase (GPX3), although the reductant for this enzyme is suggested to be protein disulfide isomerase (PDI) isoforms, which are members of the thioredoxin super-family. This proposed redox cycle is adapted from and available in more detail in the review by Meyer et al. [50][84].
The increased H2O2 levels in the ER lumen can drive signalling, most notably the initiation of the Unfolded Protein Response (UPR), which acts to mitigate against the accumulation of unfolded or misfolded proteins in the ER lumen. One branch of the UPR is mediated by a pair of ER membrane-associated bZIP transcription factors—bZIP17 and bZIP28. UPR is also activated as a consequence of environmental perturbations including exposure to heat/chilling stress, oxidative stress, salt stress, induction of immunity and senescence [45][51][52][53][79,85,86,87].
